J Korean Med Sci.
2020 Dec;35(46):e403. 10.3346/jkms.2020.35.e403.
Osteoporotic Fractures of the Spine, Hip, and Other Locations after Adjuvant Endocrine Therapy with Aromatase Inhibitors in Breast Cancer Patients: a Meta-analysis
- Affiliations
-
- 1Department of Orthopedic Surgery, Seoul National University Bundang Hospital, Seongnam, Korea
- 2Center for Breast Cancer, Research Institute and Hospital, National Cancer Center, Goyang, Korea
- 3Department of Internal Medicine, Gangneung Asan Hospital, University of Ulsan College of Medicine, Gangneung, Korea
- 4Department of Internal Medicine, Center for Thyroid Cancer, National Cancer Center, Goyang, Korea
- 5College of Pharmacy, Daegu Catholic University, Gyeongsan, Korea
- 6Department of Pharmacy, Chung-Ang University College of Pharmacy, Seoul, Korea
- 7Department of Orthopedic Surgery, Gyeongsang National University Hospital, Gyeongsang National University School of Medicine, Jinju, Korea
- KMID: 2509039
- DOI: http://doi.org/10.3346/jkms.2020.35.e403
Abstract
- Background
Aromatase inhibitors (AIs) play an important role in the endocrine therapy of postmenopausal breast cancer patients, with a recent tendency to extend the duration of their use. However, AIs may increase the risk of osteoporotic bone fractures. This meta-analysis evaluated the risk of osteoporotic fractures of the hip, spine, and other locations in breast cancer patients using AIs.
Methods
We performed a systematic search to identify randomized controlled clinical trials that investigated osteoporotic fractures in breast cancer patients on AI therapy. The main outcomes were the incidence and risk of osteoporotic fractures in general and of hip, vertebral, and non-vertebral fractures in AI users and controls.
Results
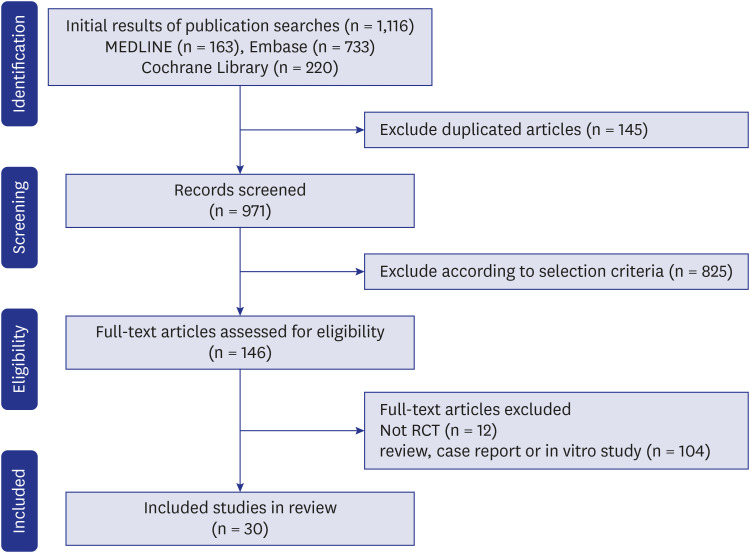
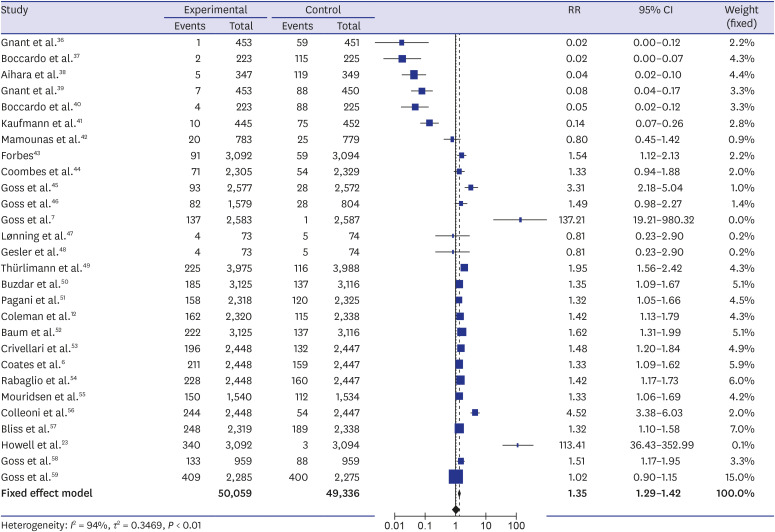
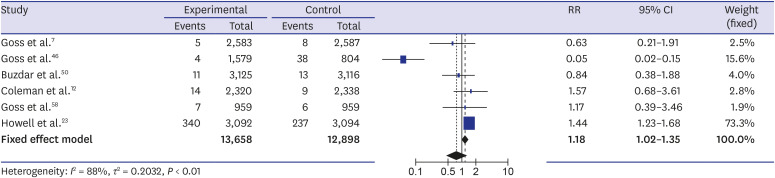
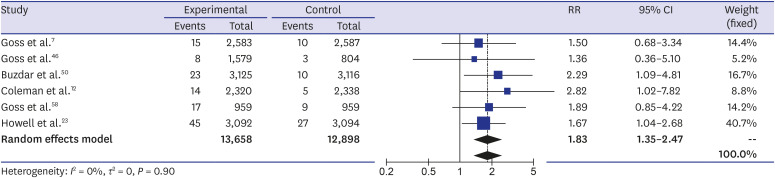
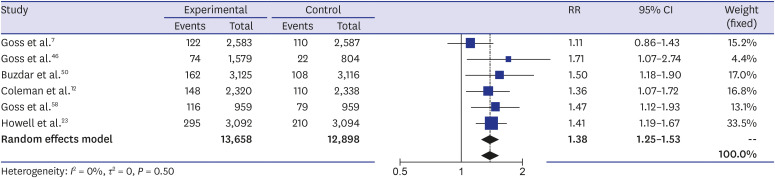
The systematic review found a total of 30 randomized controlled trials including 117,974 participants. The meta-analysis showed a higher incidence of osteoporotic fracture in AI users: The crude risk ratio for all osteoporotic fractures was 1.35 (95% confidence interval [CI], 1.29–1.42;P < 0.001), for hip fractures 1.18 (95% CI, 1.02–1.35; P < 0.001), for vertebral fractures 1.84 (95% CI, 1.36–2.49; P < 0.001), and for non-vertebral fractures 1.18 (95% CI, 1.02–1.35; P < 0.001), respectively, compared to the controls.
Conclusion
Our meta-analysis suggested an increased risk of osteoporotic fractures for AI therapy in patients with breast cancer that was most expressed for vertebral fractures. Breast cancer patients on AIs need to be monitored for osteoporosis and osteoporotic fractures, and active prevention measures should be implemented.
Keyword
Figure
Reference
-
1. Yap YS, Lu YS, Tamura K, Lee JE, Ko EY, Park YH, et al. Insights into breast cancer in the East vs the West: a review. JAMA Oncol. Forthcoming. 2019; DOI: 10.1001/jamaoncol.2019.0620.2. Howlader N, Altekruse SF, Li CI, Chen VW, Clarke CA, Ries LA, et al. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst. 2014; 106(5):dju055. PMID: 24777111.
Article3. Dowsett M, Cuzick J, Ingle J, Coates A, Forbes J, Bliss J, et al. Meta-analysis of breast cancer outcomes in adjuvant trials of aromatase inhibitors versus tamoxifen. J Clin Oncol. 2010; 28(3):509–518. PMID: 19949017.
Article4. Mauri D, Pavlidis N, Polyzos NP, Ioannidis JP. Survival with aromatase inhibitors and inactivators versus standard hormonal therapy in advanced breast cancer: meta-analysis. J Natl Cancer Inst. 2006; 98(18):1285–1291. PMID: 16985247.
Article5. Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet. 2015; 386(10001):1341–1352. PMID: 26211827.6. Coates AS, Keshaviah A, Thürlimann B, Mouridsen H, Mauriac L, Forbes JF, et al. Five years of letrozole compared with tamoxifen as initial adjuvant therapy for postmenopausal women with endocrine-responsive early breast cancer: update of study BIG 1-98. J Clin Oncol. 2007; 25(5):486–492. PMID: 17200148.
Article7. Goss PE, Ingle JN, Martino S, Robert NJ, Muss HB, Piccart MJ, et al. Randomized trial of letrozole following tamoxifen as extended adjuvant therapy in receptor-positive breast cancer: updated findings from NCIC CTG MA.17. J Natl Cancer Inst. 2005; 97(17):1262–1271. PMID: 16145047.8. Winer EP, Hudis C, Burstein HJ, Wolff AC, Pritchard KI, Ingle JN, et al. American Society of Clinical Oncology technology assessment on the use of aromatase inhibitors as adjuvant therapy for postmenopausal women with hormone receptor-positive breast cancer: status report 2004. J Clin Oncol. 2005; 23(3):619–629. PMID: 15545664.
Article9. Smith IE, Dowsett M. Aromatase inhibitors in breast cancer. N Engl J Med. 2003; 348(24):2431–2442. PMID: 12802030.
Article10. Becker T, Lipscombe L, Narod S, Simmons C, Anderson GM, Rochon PA. Systematic review of bone health in older women treated with aromatase inhibitors for early-stage breast cancer. J Am Geriatr Soc. 2012; 60(9):1761–1767. PMID: 22985145.
Article11. Lee SJ, Kim KM, Brown JK, Brett A, Roh YH, Kang DR, et al. Negative impact of aromatase inhibitors on proximal femoral bone mass and geometry in postmenopausal women with breast cancer. Calcif Tissue Int. 2015; 97(6):551–559. PMID: 26232103.
Article12. Coleman RE, Banks LM, Girgis SI, Kilburn LS, Vrdoljak E, Fox J, et al. Skeletal effects of exemestane on bone-mineral density, bone biomarkers, and fracture incidence in postmenopausal women with early breast cancer participating in the Intergroup Exemestane Study (IES): a randomised controlled study. Lancet Oncol. 2007; 8(2):119–127. PMID: 17267326.
Article13. Amir E, Seruga B, Niraula S, Carlsson L, Ocaña A. Toxicity of adjuvant endocrine therapy in postmenopausal breast cancer patients: a systematic review and meta-analysis. J Natl Cancer Inst. 2011; 103(17):1299–1309. PMID: 21743022.
Article14. Taxel P, Faircloth E, Idrees S, Van Poznak C. Cancer treatment-induced bone loss in women with breast cancer and men with prostate cancer. J Endocr Soc. 2018; 2(7):574–588. PMID: 29942922.
Article15. Melton LJ 3rd, Hartmann LC, Achenbach SJ, Atkinson EJ, Therneau TM, Khosla S. Fracture risk in women with breast cancer: a population-based study. J Bone Miner Res. 2012; 27(5):1196–1205. PMID: 22258822.
Article16. Yoo JH, Moon SH, Ha YC, Lee DY, Gong HS, Park SY, et al. Osteoporotic fracture: 2015 position statement of the Korean Society for Bone and Mineral Research. J Bone Metab. 2015; 22(4):175–181. PMID: 26713308.
Article17. Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009; 6(7):e1000097. PMID: 19621072.
Article18. Schardt C, Adams MB, Owens T, Keitz S, Fontelo P. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med Inform Decis Mak. 2007; 7(1):16. PMID: 17573961.
Article19. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011; 343:d5928. PMID: 22008217.
Article20. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994; 50(4):1088–1101. PMID: 7786990.
Article21. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997; 315(7109):629–634. PMID: 9310563.
Article22. R Core Team. R: a Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing;2020.23. Howell A. Adjuvant aromatase inhibitors for breast cancer. Lancet. 2005; 366(9484):431–433. PMID: 16084234.
Article24. Body JJ. Increased fracture rate in women with breast cancer: a review of the hidden risk. BMC Cancer. 2011; 11(1):384. PMID: 21875433.
Article25. Khosla S, Oursler MJ, Monroe DG. Estrogen and the skeleton. Trends Endocrinol Metab. 2012; 23(11):576–581. PMID: 22595550.
Article26. Venken K, Callewaert F, Boonen S, Vanderschueren D. Sex hormones, their receptors and bone health. Osteoporos Int. 2008; 19(11):1517–1525. PMID: 18392663.
Article27. Park C, Ha YC, Jang S, Jang S, Yoon HK, Lee YK. The incidence and residual lifetime risk of osteoporosis-related fractures in Korea. J Bone Miner Metab. 2011; 29(6):744–751. PMID: 21644058.
Article28. Cooper C, Atkinson EJ, Jacobsen SJ, O'Fallon WM, Melton LJ 3rd. Population-based study of survival after osteoporotic fractures. Am J Epidemiol. 1993; 137(9):1001–1005. PMID: 8317445.
Article29. Yoon HK, Park C, Jang S, Jang S, Lee YK, Ha YC. Incidence and mortality following hip fracture in Korea. J Korean Med Sci. 2011; 26(8):1087–1092. PMID: 21860561.
Article30. Edwards BJ, Raisch DW, Shankaran V, McKoy JM, Gradishar W, Bunta AD, et al. Cancer therapy associated bone loss: implications for hip fractures in mid-life women with breast cancer. Clin Cancer Res. 2011; 17(3):560–568. PMID: 21288927.
Article31. Cummings SR, Melton LJ. Epidemiology and outcomes of osteoporotic fractures. Lancet. 2002; 359(9319):1761–1767. PMID: 12049882.
Article32. Hillner BE, Ingle JN, Chlebowski RT, Gralow J, Yee GC, Janjan NA, et al. American Society of Clinical Oncology 2003 update on the role of bisphosphonates and bone health issues in women with breast cancer. J Clin Oncol. 2003; 21(21):4042–4057. PMID: 12963702.
Article33. Rizzoli R, Body JJ, DeCensi A, Reginster JY, Piscitelli P, Brandi ML, et al. Guidance for the prevention of bone loss and fractures in postmenopausal women treated with aromatase inhibitors for breast cancer: an ESCEO position paper. Osteoporos Int. 2012; 23(11):2567–2576. PMID: 22270857.
Article34. Hadji P, Aapro MS, Body JJ, Gnant M, Brandi ML, Reginster JY, et al. Management of aromatase inhibitor-associated bone loss (AIBL) in postmenopausal women with hormone sensitive breast cancer: joint position statement of the IOF, CABS, ECTS, IEG, ESCEO IMS, and SIOG. J Bone Oncol. 2017; 7:1–12. PMID: 28413771.
Article35. Baatjes KJ, Apffelstaedt JP, Kotze MJ, Conradie M. Postmenopausal breast cancer, aromatase inhibitors, and bone health: what the surgeon should know. World J Surg. 2016; 40(9):2149–2156. PMID: 27189076.
Article36. Gnant M, Mlineritsch B, Schippinger W, Luschin-Ebengreuth G, Pöstlberger S, Menzel C, et al. Endocrine therapy plus zoledronic acid in premenopausal breast cancer. N Engl J Med. 2009; 360(7):679–691. PMID: 19213681.
Article37. Boccardo F, Rubagotti A, Puntoni M, Guglielmini P, Amoroso D, Fini A, et al. Switching to anastrozole versus continued tamoxifen treatment of early breast cancer: preliminary results of the Italian Tamoxifen Anastrozole Trial. J Clin Oncol. 2005; 23(22):5138–5147. PMID: 16009955.
Article38. Aihara T, Takatsuka Y, Ohsumi S, Aogi K, Hozumi Y, Imoto S, et al. Phase III randomized adjuvant study of tamoxifen alone versus sequential tamoxifen and anastrozole in Japanese postmenopausal women with hormone-responsive breast cancer: N-SAS BC03 study. Breast Cancer Res Treat. 2010; 121(2):379–387. PMID: 20390343.
Article39. Gnant M, Mlineritsch B, Stoeger H, Luschin-Ebengreuth G, Heck D, Menzel C, et al. Adjuvant endocrine therapy plus zoledronic acid in premenopausal women with early-stage breast cancer: 62-month follow-up from the ABCSG-12 randomised trial. Lancet Oncol. 2011; 12(7):631–641. PMID: 21641868.
Article40. Boccardo F, Guglielmini P, Bordonaro R, Fini A, Massidda B, Porpiglia M, et al. Switching to anastrozole versus continued tamoxifen treatment of early breast cancer: long term results of the Italian Tamoxifen Anastrozole trial. Eur J Cancer. 2013; 49(7):1546–1554. PMID: 23415888.
Article41. Kaufmann M, Jonat W, Hilfrich J, Eidtmann H, Gademann G, Zuna I, et al. Improved overall survival in postmenopausal women with early breast cancer after anastrozole initiated after treatment with tamoxifen compared with continued tamoxifen: the ARNO 95 Study. J Clin Oncol. 2007; 25(19):2664–2670. PMID: 17563395.
Article42. Mamounas EP, Jeong JH, Wickerham DL, Smith RE, Ganz PA, Land SR, et al. Benefit from exemestane as extended adjuvant therapy after 5 years of adjuvant tamoxifen: intention-to-treat analysis of the National Surgical Adjuvant Breast And Bowel Project B-33 trial. J Clin Oncol. 2008; 26(12):1965–1971. PMID: 18332472.
Article43. Forbes JF. The use of early adjuvant aromatase inhibitor therapy: contributions from the BIG 1-98 letrozole trial. Semin Oncol. 2006; 33(2 Suppl 7):S2–S7.
Article44. Coombes RC, Hall E, Gibson LJ, Paridaens R, Jassem J, Delozier T, et al. A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. N Engl J Med. 2004; 350(11):1081–1092. PMID: 15014181.
Article45. Goss PE, Ingle JN, Martino S, Robert NJ, Muss HB, Piccart MJ, et al. A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. N Engl J Med. 2003; 349(19):1793–1802. PMID: 14551341.
Article46. Goss PE, Ingle JN, Pater JL, Martino S, Robert NJ, Muss HB, et al. Late extended adjuvant treatment with letrozole improves outcome in women with early-stage breast cancer who complete 5 years of tamoxifen. J Clin Oncol. 2008; 26(12):1948–1955. PMID: 18332475.
Article47. Lønning PE, Geisler J, Krag LE, Erikstein B, Bremnes Y, Hagen AI, et al. Effects of exemestane administered for 2 years versus placebo on bone mineral density, bone biomarkers, and plasma lipids in patients with surgically resected early breast cancer. J Clin Oncol. 2005; 23(22):5126–5137. PMID: 15983390.
Article48. Geisler J, Lønning PE, Krag LE, Løkkevik E, Risberg T, Hagen AI, et al. Changes in bone and lipid metabolism in postmenopausal women with early breast cancer after terminating 2-year treatment with exemestane: a randomised, placebo-controlled study. Eur J Cancer. 2006; 42(17):2968–2975. PMID: 16963261.
Article49. Breast International Group (BIG) 1-98 Collaborative Group. Thürlimann B, Keshaviah A, Coates AS, Mouridsen H, Mauriac L, et al. A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N Engl J Med. 2005; 353(26):2747–2757. PMID: 16382061.
Article50. Buzdar AU. ATAC trialists' group. ‘Arimidex’ (anastrozole) versus tamoxifen as adjuvant therapy in postmenopausal women with early breast cancer--efficacy overview. J Steroid Biochem Mol Biol. 2003; 86(3-5):399–403. PMID: 14623537.
Article51. Pagani O, Regan MM, Walley BA, Fleming GF, Colleoni M, Láng I, et al. Adjuvant exemestane with ovarian suppression in premenopausal breast cancer. N Engl J Med. 2014; 371(2):107–118. PMID: 24881463.52. Baum M, Buzdar A. The current status of aromatase inhibitors in the management of breast cancer. Surg Clin North Am. 2003; 83(4):973–994. PMID: 12875605.
Article53. Crivellari D, Sun Z, Coates AS, Price KN, Thürlimann B, Mouridsen H, et al. Letrozole compared with tamoxifen for elderly patients with endocrine-responsive early breast cancer: the BIG 1-98 trial. J Clin Oncol. 2008; 26(12):1972–1979. PMID: 18332471.
Article54. Rabaglio M, Sun Z, Price KN, Castiglione-Gertsch M, Hawle H, Thürlimann B, et al. Bone fractures among postmenopausal patients with endocrine-responsive early breast cancer treated with 5 years of letrozole or tamoxifen in the BIG 1-98 trial. Ann Oncol. 2009; 20(9):1489–1498. PMID: 19474112.
Article55. BIG 1-98 Collaborative Group, Mouridsen H, Giobbie-Hurder A, Goldhirsch A, Thürlimann B, Paridaens R, et al. Letrozole therapy alone or in sequence with tamoxifen in women with breast cancer. N Engl J Med. 2009; 361(8):766–776. PMID: 19692688.
Article56. Colleoni M, Giobbie-Hurder A, Regan MM, Thürlimann B, Mouridsen H, Mauriac L, et al. Analyses adjusting for selective crossover show improved overall survival with adjuvant letrozole compared with tamoxifen in the BIG 1-98 study. J Clin Oncol. 2011; 29(9):1117–1124. PMID: 21321298.
Article57. Bliss JM, Kilburn LS, Coleman RE, Forbes JF, Coates AS, Jones SE, et al. Disease-related outcomes with long-term follow-up: an updated analysis of the intergroup exemestane study. J Clin Oncol. 2012; 30(7):709–717. PMID: 22042946.
Article58. Goss PE, Ingle JN, Pritchard KI, Robert NJ, Muss H, Gralow J, et al. Extending aromatase-inhibitor adjuvant therapy to 10 years. N Engl J Med. 2016; 375(3):209–219. PMID: 27264120.
Article59. Goss PE, Ingle JN, Alés-Martínez JE, Cheung AM, Chlebowski RT, Wactawski-Wende J, et al. Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med. 2011; 364(25):2381–2391. PMID: 21639806.
Article60. Neuner JM, Yen TW, Sparapani RA, Laud PW, Nattinger AB. Fracture risk and adjuvant hormonal therapy among a population-based cohort of older female breast cancer patients. Osteoporos Int. 2011; 22(11):2847–2855. PMID: 21170643.
Article61. Jakesz R, Jonat W, Gnant M, Mittlboeck M, Greil R, Tausch C, et al. Switching of postmenopausal women with endocrine-responsive early breast cancer to anastrozole after 2 years' adjuvant tamoxifen: combined results of ABCSG trial 8 and ARNO 95 trial. Lancet. 2005; 366(9484):455–462. PMID: 16084253.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Endocrine Therapy for Breast Cancer
- Current Understanding of Endocrine Therapy for Breast Cancer
- Risk of Osteoporotic Fracture in Patients with Breast Cancer: Meta-Analysis
- Medical Treatment of Early Breast Cancer
- Survival Benefit of Zoledronic Acid in Postmenopausal Breast Cancer Patients Receiving Aromatase Inhibitors