J Korean Neurosurg Soc.
2020 Nov;63(6):821-826. 10.3340/jkns.2020.0020.
Extent of Hyperostotic Bone Resection in Convexity Meningioma to Achieve Pathologically Free Margins
- Affiliations
-
- 1Division of Neurosurgery, Cairo University Hospitals, Cairo, Egypt
- 2Division of Neurosurgery, Fayoum University, Fayoum, Egypt
- KMID: 2508613
- DOI: http://doi.org/10.3340/jkns.2020.0020
Abstract
Objective
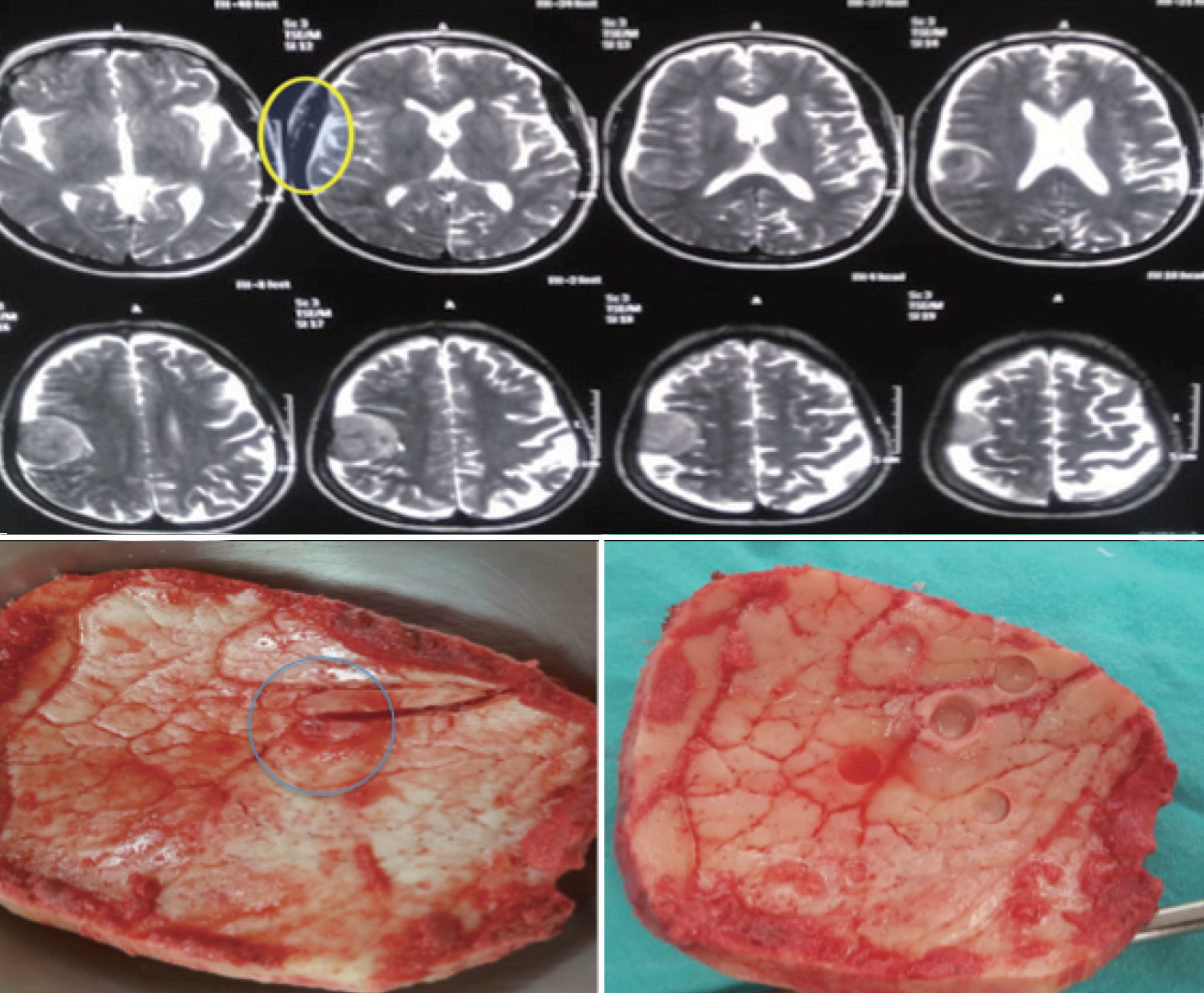
: Hyperostosis in meningiomas can be present in 4.5% to 44% of cases. Radical resection should include aggressive removal of invaded bone. It is not clear however to what extent bone removal should be carried to achieve pathologically free margins, especially that in many cases, there is a T2 hyperintense signal that extends beyond the hyperostotic bone. In this study we try to investigate the perimeter of tumour cells outside the visible nidus of hyperostotic bone and to what extent they are present outside this nidus. This would serve as an initial step for setting guidelines on dealing with hyperostosis in meningioma surgery.
Methods
: This is a prospective case series that included 14 patients with convexity meningiomas and hyperostosis during the period from March 2017 to August 2018 in two university hospitals. Patients demographics, clinical, imaging characteristics, intraoperative and postoperative data were collected and analysed. In all cases, all visible abnormal bone was excised bearing in mind to also include the hyperintense diploe in magnetic resonance imaging (MRI) T2 weighted images after careful preoperative assessment. To examine bony tumour invasion, five marked bone biopsies were taken from the craniotomy flap for histopathological examinations. These include one from the centre of hyperostotic nidus and the other four from the corners at a 2-cm distance from the margin of the nidus.
Results
: Our study included five males (35.7%) and nine females (64.3%) with a mean age of 43.75 years (33-55). Tumor site was parietal in seven cases (50%), fronto-parietal in three cases (21.4%), parieto-occipital in two cases (14.2%), frontal region in one case and bicoronal (midline) in one case. Tumour pathology revealed a World Health Organization (WHO) grade I in seven cases (50%), atypical meningioma (WHO II) in five cases (35.7%) and anaplastic meningioma (WHO III) in two cases (14.2%). In all grade I and II meningiomas, bone biopsies harvested from the nidus revealed infiltration with tumour cells while all other bone biopsies from the four corners (2 cm from nidus) were free. In cases of anaplastic meningiomas, all five biopsies were positive for tumour cells.
Conclusion
: Removal of the gross epicentre of hyperostotic bone with the surrounding 2 cm is adequate to ensure radical excision and free bone margins in grade I and II meningiomas. Hyperintense signal change in MRI T2 weighted images, even beyond visible hypersototic areas, doesn’t necessarily represent tumour invasion.
Keyword
Figure
Reference
-
References
1. Alzhrani G, Couldwell W. Bony hyperostosis recurrence after complete resection of sphenoorbital meningioma. Cureus. 9:e1540. 2017.
Article2. Banu MA, Szentirmai O, Mascarenhas L, Salek AA, Anand VK, Schwartz TH. Pneumocephalus patterns following endonasal endoscopic skull base surgery as predictors of postoperative CSF leaks. J Neurosurg. 121:961–975. 2014.
Article3. Bonnal J, Thibaut A, Brotchi J, Born J. Invading meningiomas of the sphenoid ridge. J Neurosurg. 53:587–599. 1980.
Article4. Di Cristofori A, Del Bene M, Locatelli M, Boggio F, Ercoli G, Ferrero S, et al. Meningioma and bone hyperostosis: expression of bone stimulating factors and review of the literature. World Neurosurg. 115:e774–e781. 2018.
Article5. Derome PJ, Guiot G. Bone problems in meningiomas invading the base of the skull. Clin Neurosurg. 25:435–451. 1978.
Article6. Dziuk TW, Woo S, Butler EB, Thornby J, Grossman R, Dennis WS, et al. Malignant meningioma: an indication for initial aggressive surgery and adjuvant radiotherapy. J Neurooncol. 37:177–188. 1998.7. Gabeau-Lacet D, Mohapatra G, Betensky R, Barker F, Loeffler J, Louis D. Bone involvement predicts poor outcome in atypical meningioma. Int J Radiat Oncol Biol Phys. 72:S207. 2008.
Article8. Goyal LK, Suh JH, Mohan DS, Prayson RA, Lee J, Barnett GH. Local control and overall survival in atypical meningioma: a retrospective study. Int J Radiat Oncol Biol Phys. 46:57–61. 2000.
Article9. Goyal N, Kakkar A, Sarkar C, Agrawal D. Does bony hyperostosis in intracranial meningioma signify tumor invasion? A radio-pathologic study. Neurol India. 60:50–54. 2012.
Article10. Lau BL, Che Othman MI, Fathil MFMD, Liew DNS, Lim SS, Bujang MA, et al. Does putting back hyperostotic bone flap in meningioma surgery cause tumor recurrence? An observational prospective study. World Neurosurg. 127:e497–e502. 2019.
Article11. Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 131:803–820. 2016.
Article12. Marbacher S, Coluccia D, Fathi AR, Andereggen L, Beck J, Fandino J. Intraoperative patient-specific reconstruction of partial bone flap defects after convexity meningioma resection. World Neurosurg. 79:124–130. 2013.
Article13. Milker-Zabel S, Zabel A, Schulz-Ertner D, Schlegel W, Wannenmacher M, Debus J. Fractionated stereotactic radiotherapy in patients with benign or atypical intracranial meningioma: long-term experience and prognostic factors. Int J Radiat Oncol Biol Phys. 61:809–816. 2005.
Article14. Nasseri K, Mills JR. Epidemiology of primary brain tumors in the Middle Eastern population in California, USA 2001-2005. Cancer Detect Prev. 32:363–371. 2009.
Article15. Palma L, Celli P, Franco C, Cervoni L, Cantore G. Long-term prognosis for atypical and malignant meningiomas: a study of 71 surgical cases. Neurosurg Focus. 2:E3. 1997.
Article16. Pieper DR, Al-Mefty O, Hanada Y, Buechner D. Hyperostosis associated with meningioma of the cranial base: secondary changes or tumor invasion. Neurosurgery. 44:742–747. 1999.
Article17. Simpson D. The recurrence of intracranial meningiomas after surgical treatment. J Neurol Neurosurg Psychiatry. 20:22–39. 1957.
Article18. Willis J, Smith C, Ironside JW, Erridge S, Whittle IR, Everington D. The accuracy of meningioma grading: a 10-year retrospective audit. Neuropathol Appl Neurobiol. 31:141–149. 2005.
Article19. Zwirner K, Paulsen F, Schittenhelm J, Gepfner-Tuma I, Tabatabai G, Behling F, et al. Integrative assessment of brain and bone invasion in meningioma patients. Radiat Oncol. 14:132. 2019.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Extracranial Extension of Intracranial Atypical Meningioma En Plaque with Osteoblastic Change of the Skull
- Massive Hyperostotic Meningioma En Plaque Mimicking Fibrous Dysplasia
- Convexity Meningioma En Plaque Presenting with Diffuse Hyperostosis of the Skull
- Anaplastic Cystic Meningioma
- Prognostic Factors of Atypical Meningioma: Overall Survival Rate and Progression Free Survival Rate