Neurointervention.
2020 Nov;15(3):107-116. 10.5469/neuroint.2020.00150.
Canine Model for Selective and Superselective Cerebral Intra-Arterial Therapy Testing
- Affiliations
-
- 1Department of Neurosurgery, Baylor College of Medicine, Houston, TX, USA
- 2Department of Surgery, Baylor College of Medicine, Houston, TX, USA
- 3Department of Radiology, Baylor College of Medicine, Houston, TX, USA
- 4Center for Comparative Medicine, Baylor College of Medicine, Houston, TX, USA
- KMID: 2508062
- DOI: http://doi.org/10.5469/neuroint.2020.00150
Abstract
- Purpose
With advancing endovascular technology and increasing interest in minimally invasive intra-arterial therapies such as stem cell and chemotherapy for cerebral disease, the establishment of a translational model with cerebral circulation accessible to microcatheters is needed. We report our experience catheterizing canine cerebral circulation with microcatheters, present high-resolution angiographic images of the canine vascular anatomy, describe arterial branch flow patterns and provide measurements of canine arterial conduits.
Materials and Methods
Angiograms were performed on 10 intact purpose-bred hounds. Angiography, measurements of arterial conduits and catheterization information for intracranial arterial branches were obtained.
Results
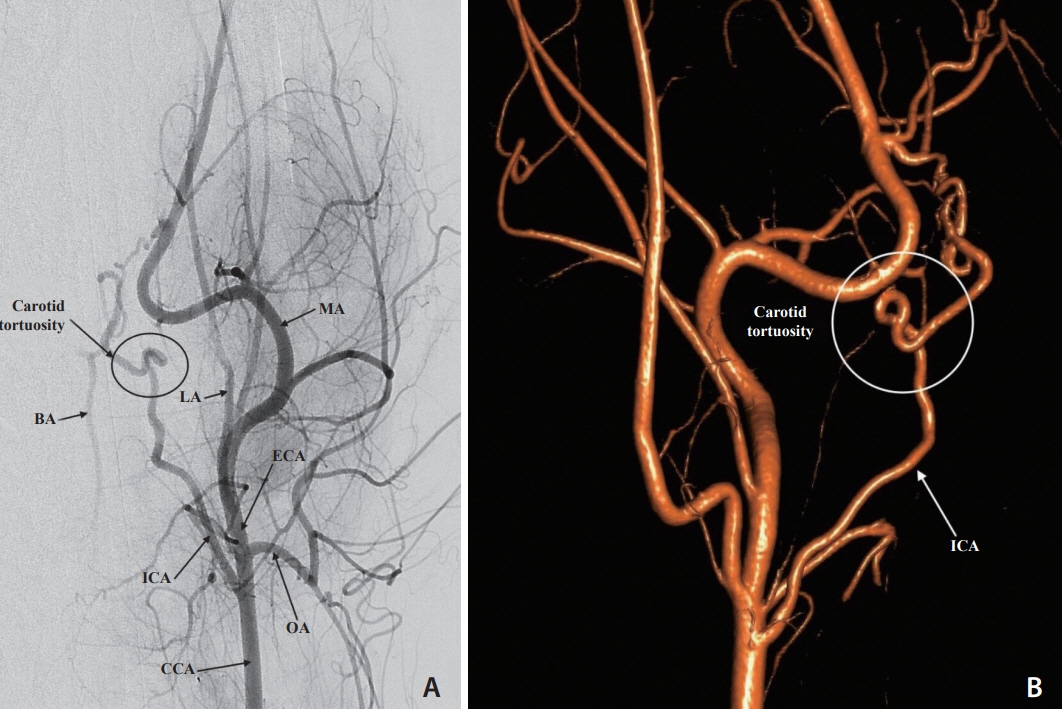
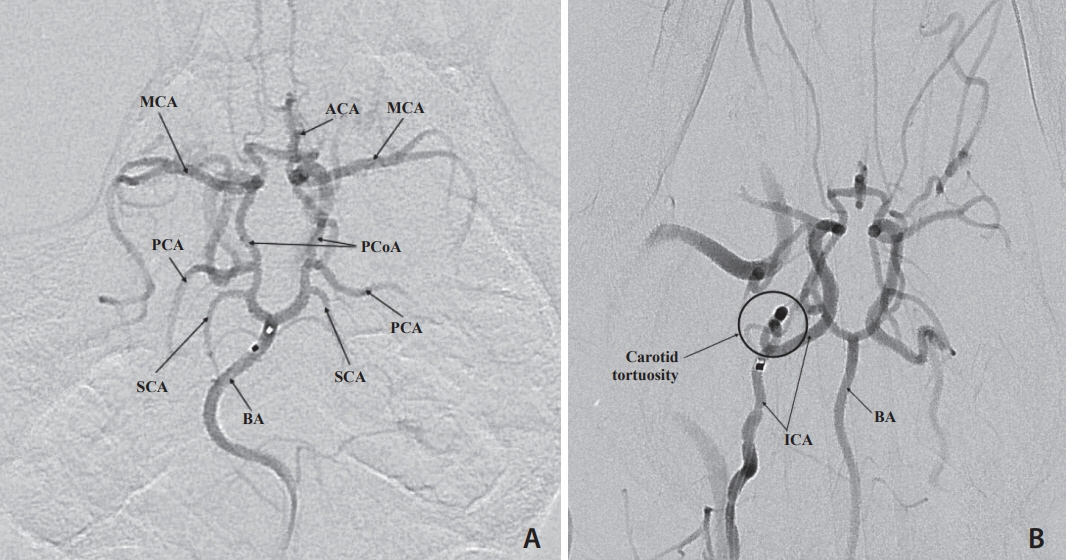
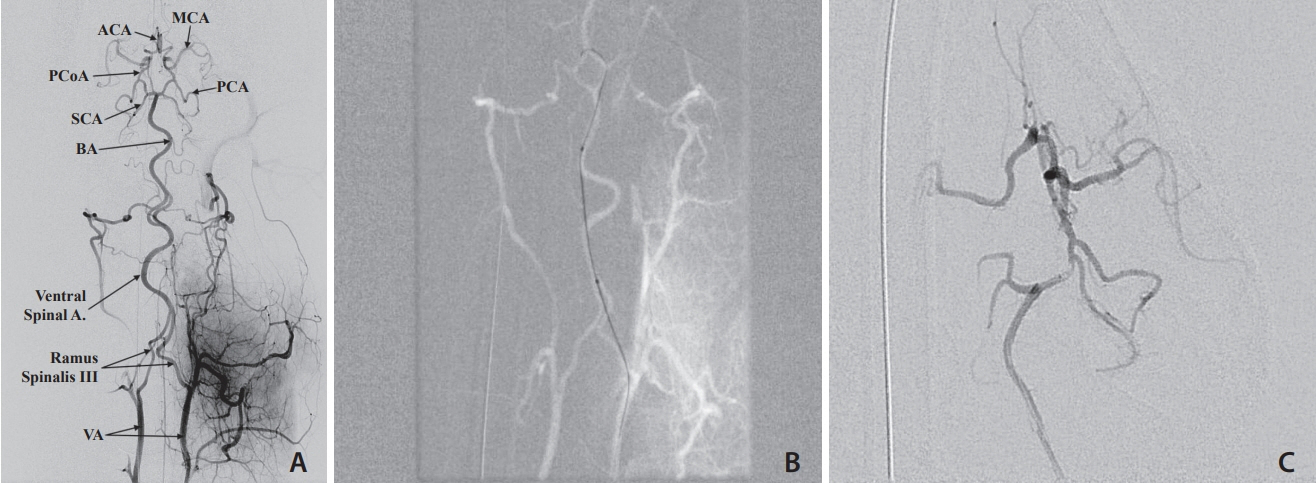
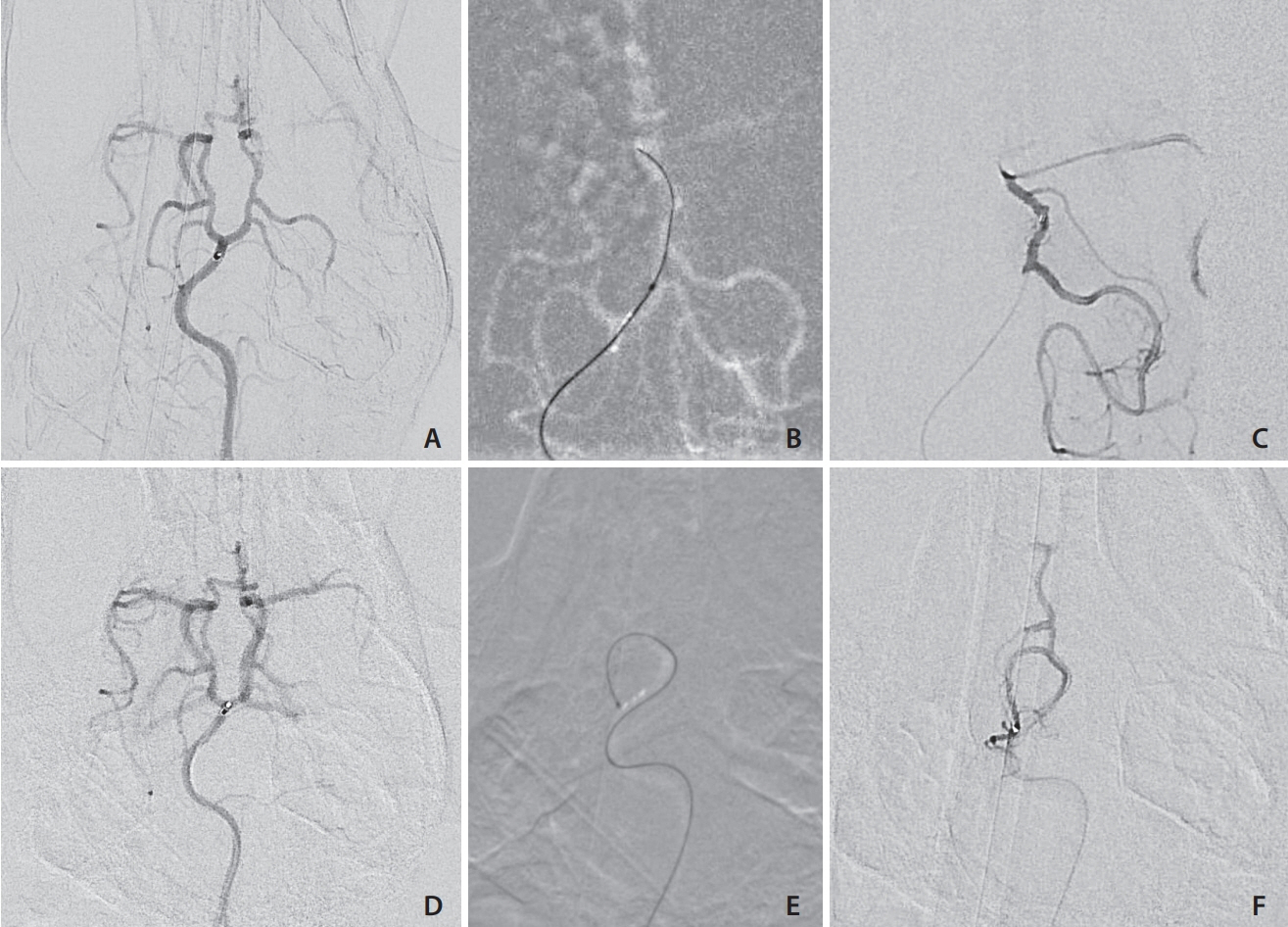
Selective and superselective cerebral angiography was successful in all subjects. Relevant arterial mean diameters include the femoral (4.64 mm), aorta (9.38 mm), external carotid (3.65 mm), internal carotid arteries (1.6 mm), vertebrobasilar system and Circle of Willis branches. Catheterization of the Circle of Willis was achieved via the posterior circulation in all subjects tested (n=3) and the use of flow directed microcatheters resulted in reduced arterial tree deformation and improved superselection of intracranial vessels. Catheterization of the intracranial circulation was attempted but not achieved via the internal carotid artery (n=7) due to its tortuosity and subsequent catheter related vasospasm.
Conclusion
The canine cerebral vasculature is posterior circulation dominant. Anterior circulation angiography is achievable via the internal carotid artery, but direct cerebral arterial access is best achieved via the posterior circulation using flow-directed microcatheters. It is feasible to deliver intra-arterial therapies to selective vascular territories within the canine cerebral circulation, thus making it a viable animal model for testing novel intra-arterial cerebral treatments.
Figure
Reference
-
1. Guzman R, Janowski M, Walczak P. Intra-arterial delivery of cell therapies for stroke. Stroke. 2018; 49:1075–1082.
Article2. Misra V, Lal A, El Khoury R, Chen PR, Savitz SI. Intra-arterial delivery of cell therapies for stroke. Stem Cells Dev. 2012; 21:1007–1015.
Article3. Moniche F, Escudero I, Zapata-Arriaza E, Usero-Ruiz M, Prieto-León M, de la Torre J, et al. Intra-arterial bone marrow mononuclear cells (BM-MNCs) transplantation in acute ischemic stroke (IBIS trial): protocol of a phase II, randomized, dose-finding, controlled multicenter trial. Int J Stroke. 2015; 10:1149–1152.
Article4. Silachev DN, Plotnikov EY, Babenko VA, Danilina TI, Zorov LD, Pevzner IB, et al. Intra-arterial administration of multipotent mesenchymal stromal cells promotes functional recovery of the brain after traumatic brain injury. Bull Exp Biol Med. 2015; 159:528–533.
Article5. Spiliopoulos S, Festas G, Theodosis A, Palialexis K, Reppas L, Konstantos C, et al. Incidence and endovascular treatment of severe spontaneous non-cerebral bleeding: a single-institution experience. Eur Radiol. 2019; 29:3296–3307.
Article6. Atchaneeyasakul K, Guada L, Ramdas K, Watanabe M, Bhattacharya P, Raval AP, et al. Large animal canine endovascular ischemic stroke models: a review. Brain Res Bull. 2016; 127:134–140.
Article7. Bouzeghrane F, Naggara O, Kallmes DF, Berenstein A, Raymond J; International Consortium of Neuroendovascular Centres. In vivo experimental intracranial aneurysm models: a systematic review. AJNR Am J Neuroradiol. 2010; 31:418–423.
Article8. Raymond J, Guilbert F, Metcalfe A, Gévry G, Salazkin I, Robledo O. Role of the endothelial lining in recurrences after coil embolization: prevention of recanalization by endothelial denudation. Stroke. 2004; 35:1471–1475.9. Shin YS, Niimi Y, Yoshino Y, Song JK, Silane M, Berenstein A. Creation of four experimental aneurysms with different hemodynamics in one dog. AJNR Am J Neuroradiol. 2005; 26:1764–1767.10. Tilley L, Smith F, Oyama M, Sleeper M. Manual of canine and feline cardiology. 4th ed. St Louis: Saunders;2008.11. Zhang Y, Jin M, Du B, Lin H, Xu C, Jiang W, et al. A novel canine model of acute vertebral artery occlusion. PLoS One. 2015; 10:e0142251.
Article12. Mawad ME, Mawad JK, Cartwright J Jr, Gokaslan Z. Long-term histopathologic changes in canine aneurysms embolized with Guglielmi detachable coils. AJNR Am J Neuroradiol. 1995; 16:7–13.13. Zu QQ, Liu S, Xu XQ, Lu SS, Sun L, Shi HB. An endovascular canine stroke model: middle cerebral artery occlusion with autologous clots followed by ipsilateral internal carotid artery blockade. Lab Invest. 2013; 93:760–767.
Article14. Lang FF, Conrad C, Gomez-Manzano C, Yung WKA, Sawaya R, Weinberg JS, et al. Phase I study of DNX-2401 (delta-24-RGD) oncolytic adenovirus: replication and immunotherapeutic effects in recurrent malignant glioma. J Clin Oncol. 2018; 36:1419–1427.
Article15. Srinivasan VM, Chintalapani G, Camstra KM, Effendi ST, Cherian J, Johnson JN, et al. Fast acquisition cone-beam computed tomography: initial experience with a 10 S protocol. J Neurointerv Surg. 2018; 10:916–920.
Article16. Cloft HJ, Altes TA, Marx WF, Raible RJ, Hudson SB, Helm GA, et al. Endovascular creation of an in vivo bifurcation aneurysm model in rabbits. Radiology. 1999; 213:223–228.
Article17. Wallace MJ, Ahrar K, Wright KC. Validation of US-guided percutaneous venous access and manual compression for studies in swine. J Vasc Interv Radiol. 2003; 14:481–483.
Article18. Boulos AS, Deshaies EM, Dalfino JC, Feustel PJ, Popp AJ, Drazin D. Tamoxifen as an effective neuroprotectant in an endovascular canine model of stroke. J Neurosurg. 2011; 114:1117–1126.
Article19. Lu SS, Liu S, Zu QQ, Xu XQ, Yu J, Wang JW, et al. In vivo MR imaging of intraarterially delivered magnetically labeled mesenchymal stem cells in a canine stroke model. PLoS One. 2013; 8:e54963.
Article20. Christoforidis GA, Rink C, Kontzialis MS, Mohammad Y, Koch RM, Abduljalil AM, et al. An endovascular canine middle cerebral artery occlusion model for the study of leptomeningeal collateral recruitment. Invest Radiol. 2011; 46:34–40.
Article21. Rink C, Christoforidis G, Abduljalil A, Kontzialis M, Bergdall V, Roy S, et al. Minimally invasive neuroradiologic model of preclinical transient middle cerebral artery occlusion in canines. Proc Natl Acad Sci U S A. 2008; 105:14100–14105.
Article22. Ellis JA, Cooke J, Singh-Moon RP, Wang M, Bruce JN, Emala CW, et al. Safety, feasibility, and optimization of intra-arterial mitoxantrone delivery to gliomas. J Neurooncol. 2016; 130:449–454.
Article23. Peschillo S, Caporlingua A, Diana F, Caporlingua F, Delfini R. New therapeutic strategies regarding endovascular treatment of glioblastoma, the role of the blood-brain barrier and new ways to bypass it. J Neurointerv Surg. 2016; 8:1078–1082.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The clinical usefulness of interventional thrombolytic therapy in acute middle cerebral artery infarction with early CT signs
- Superselective Intra-arterial Fibrinolysis for Acute Cerebral Ischemic Infarct: Usefulness of Diffusion Weighted MR Imaging1
- Atypical Developmental Venous Anomaly Associated with Single Arteriovenous Fistula and Intracerebral Hemorrhage: a Case Demonstrated by Superselective Angiography
- Transcatheter arterial embolization for congenital renal arteriovenous fistula
- Cerebral Arterial Embolism Treated by Intra-Arterial Infusion of Urokinase Which Was Occurred during Percutaneous Balloon Mitral Valvuloplasty