Korean J Physiol Pharmacol.
2020 Nov;24(6):441-452. 10.4196/kjpp.2020.24.6.441.
Trends in the development of human stem cell-based non-animal drug testing models
- Affiliations
-
- 1Department of Predictive Toxicology, Korea Institute of Toxicology, Korea Research Institute of Chemical Technology, Daejeon 34114, Korea
- KMID: 2507727
- DOI: http://doi.org/10.4196/kjpp.2020.24.6.441
Abstract
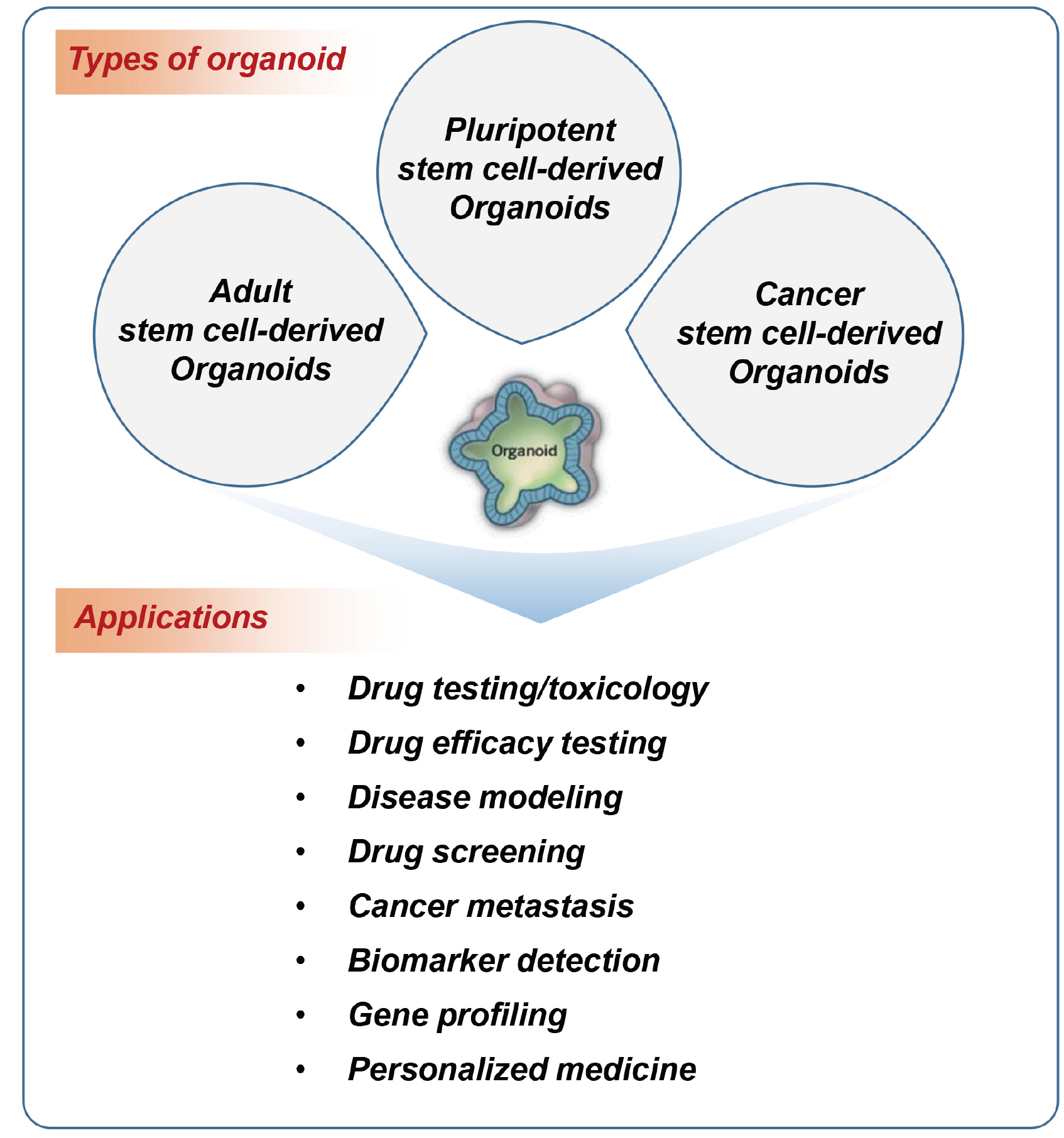
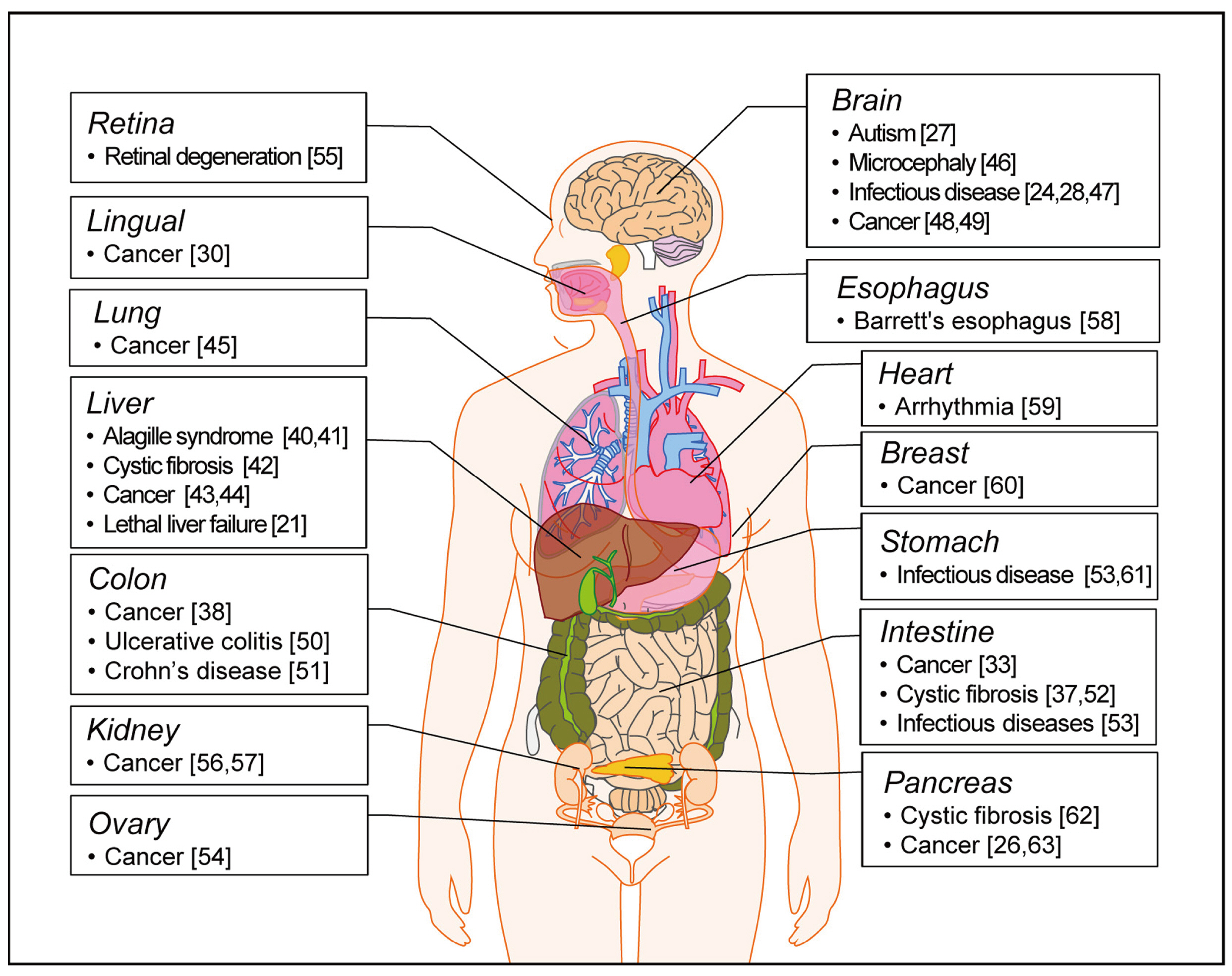
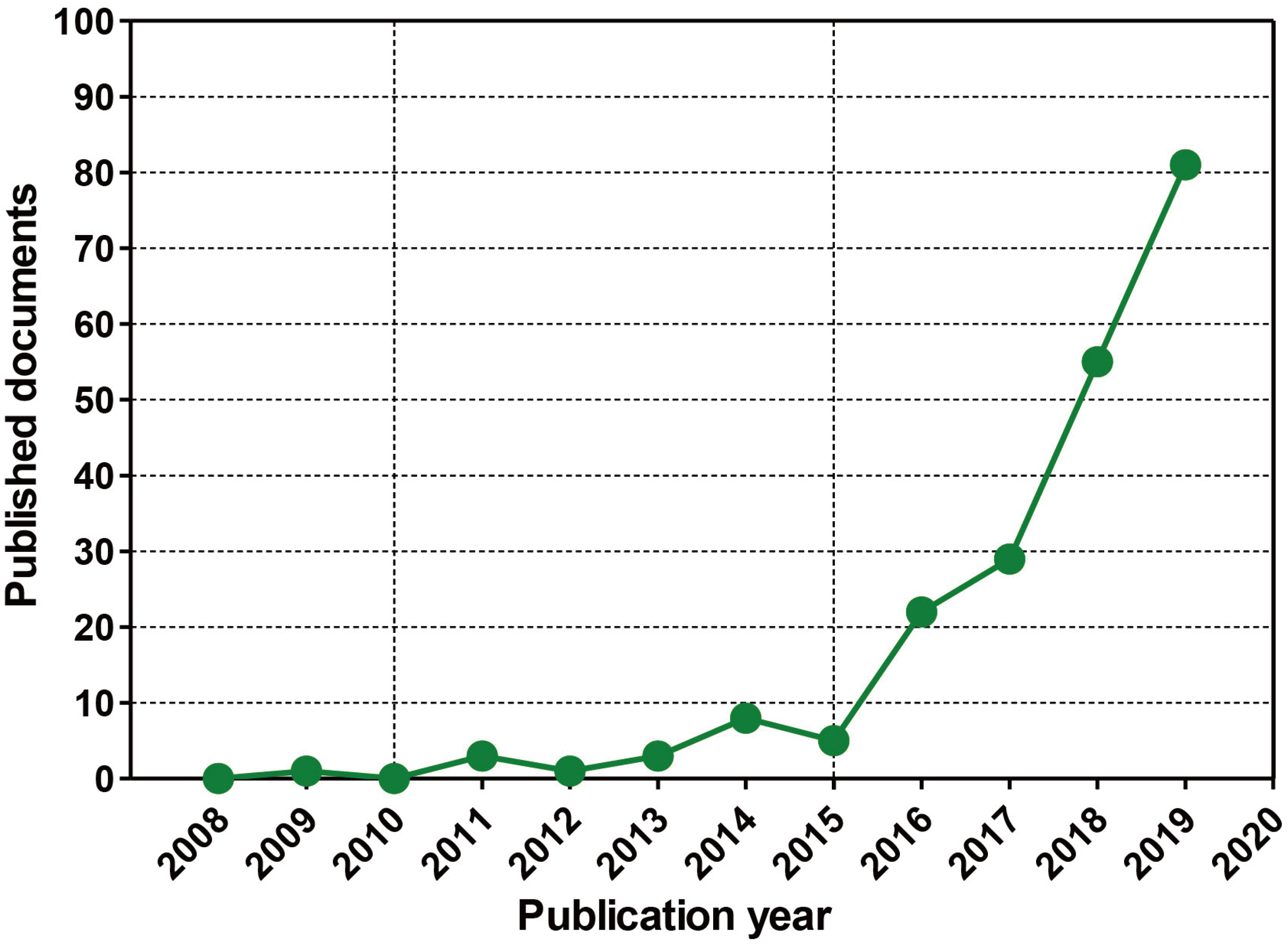
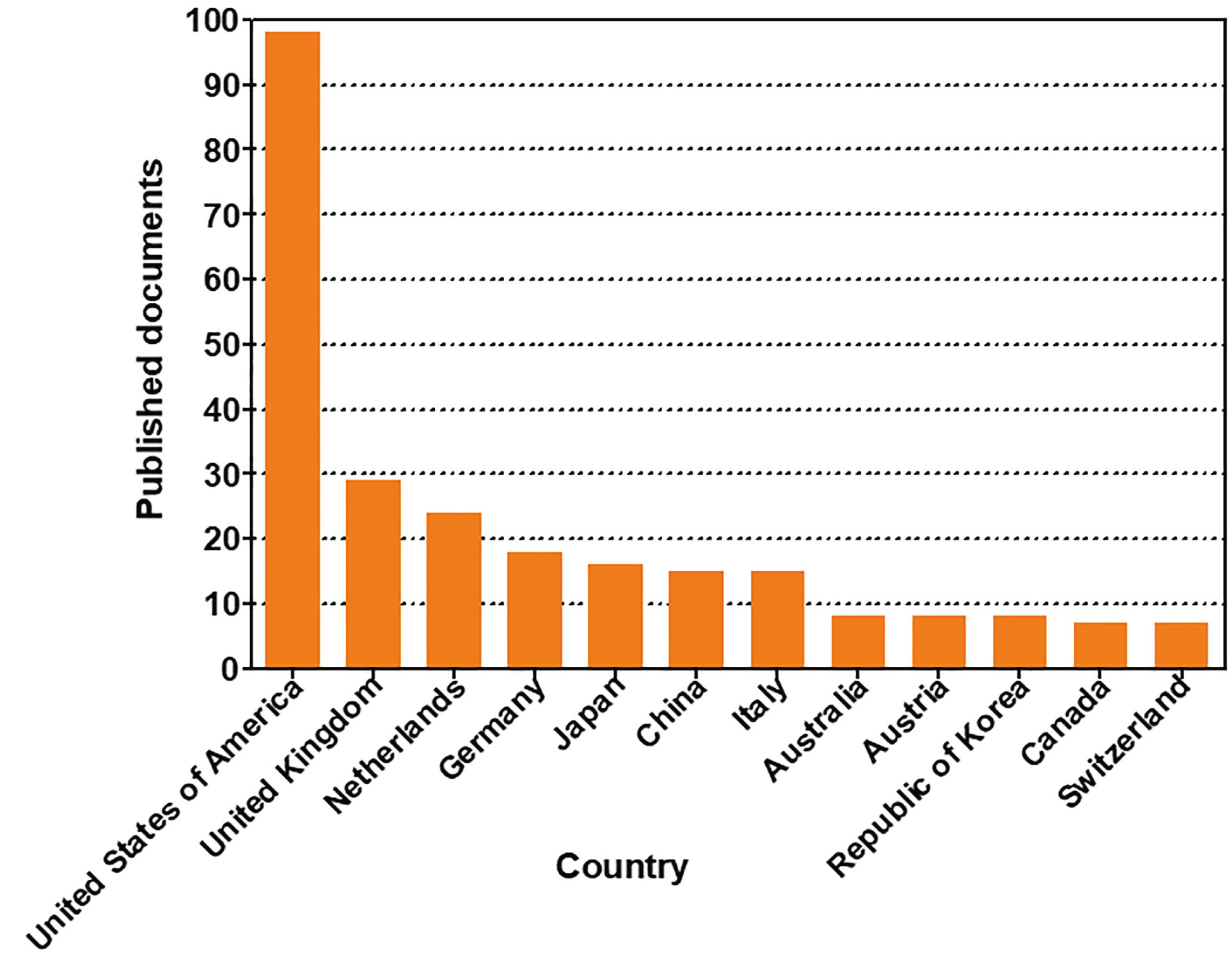
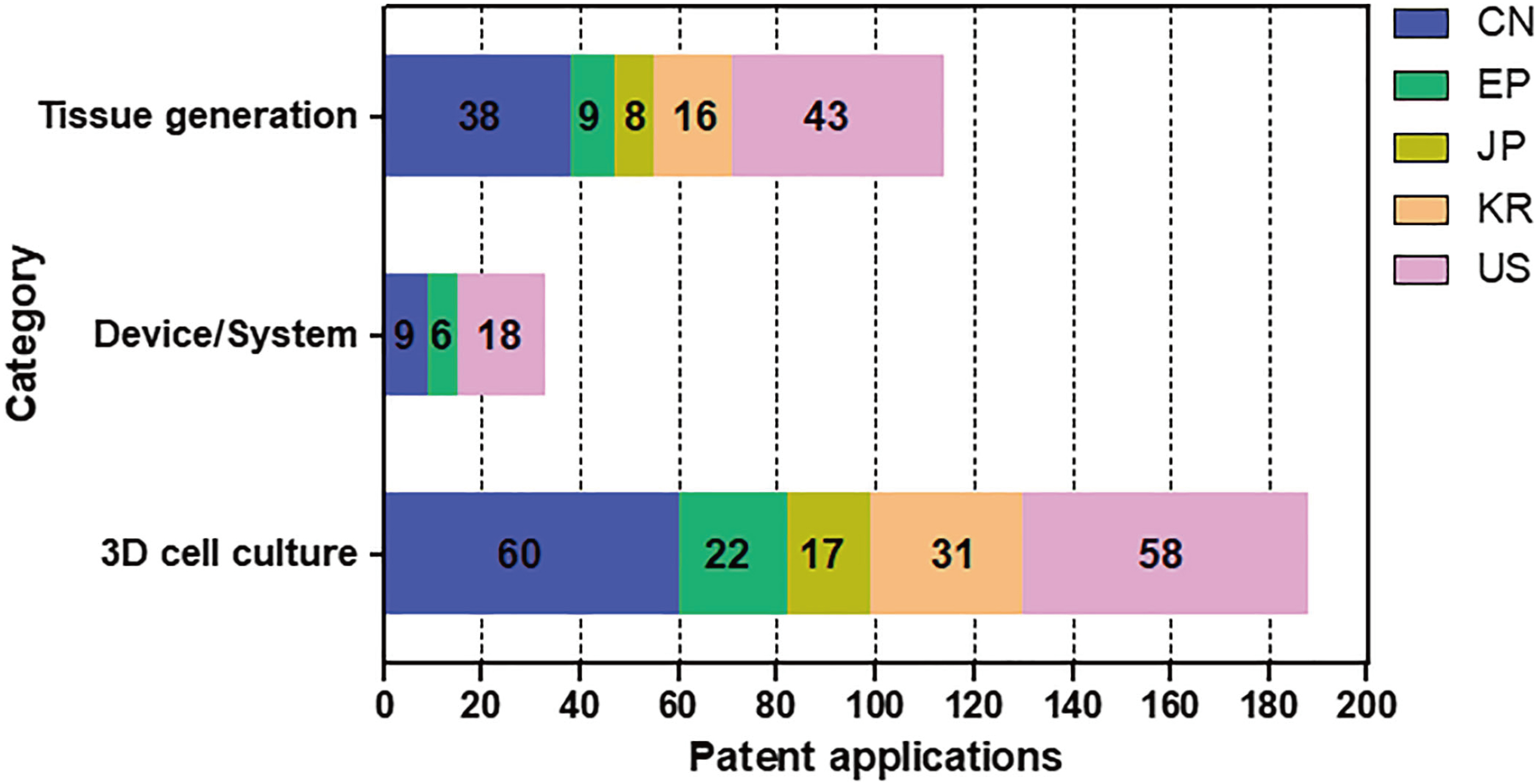
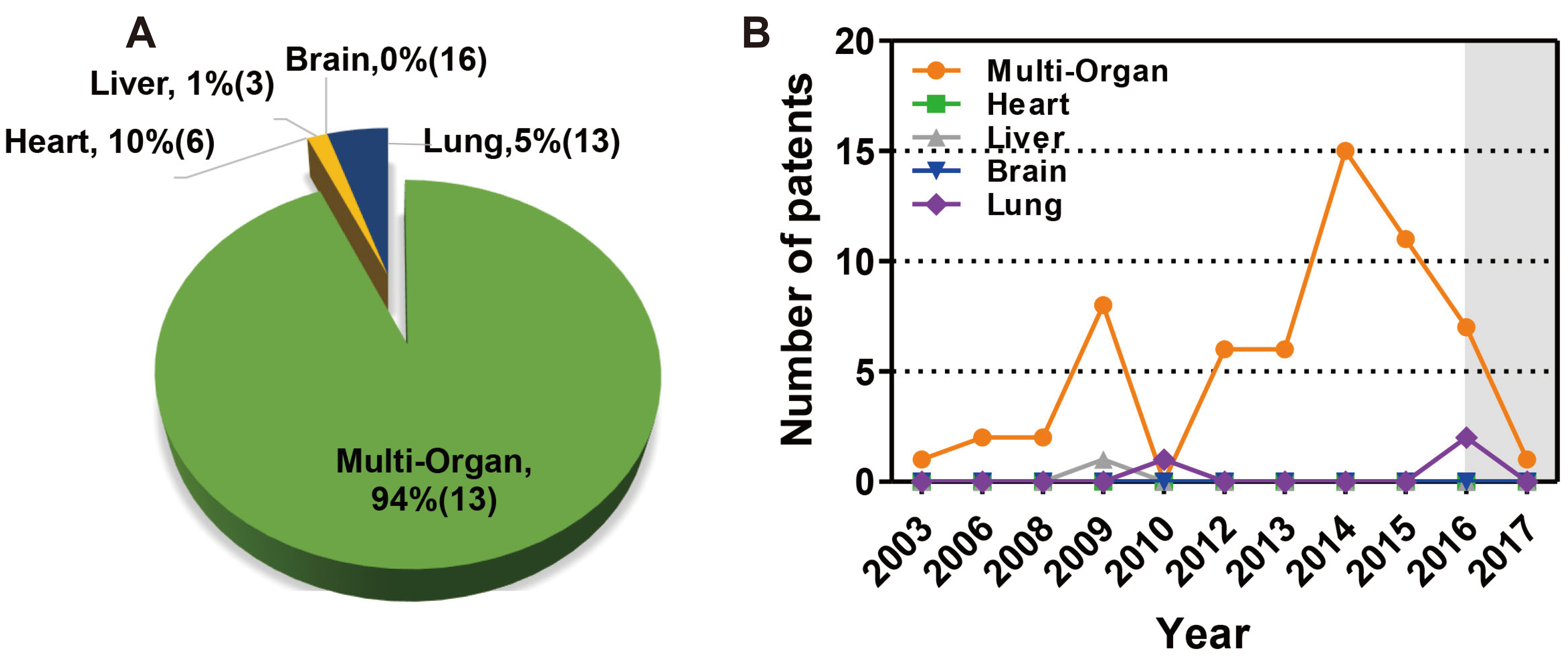
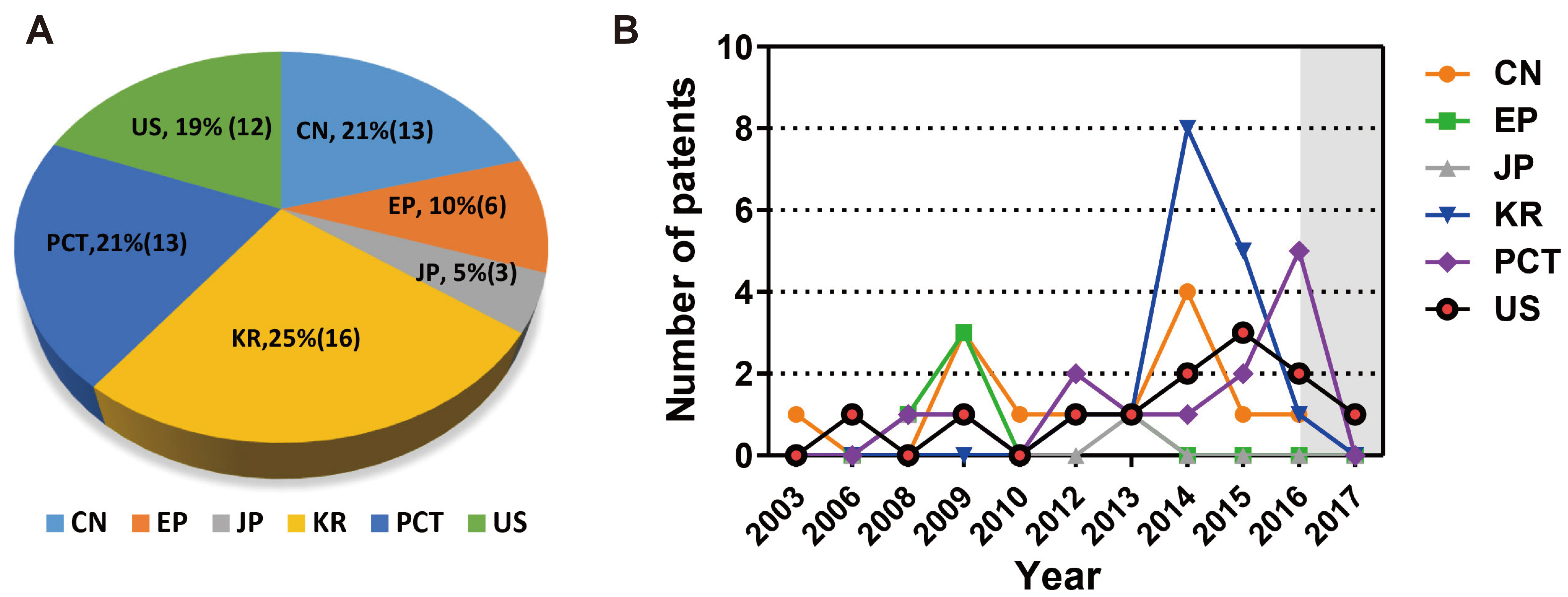
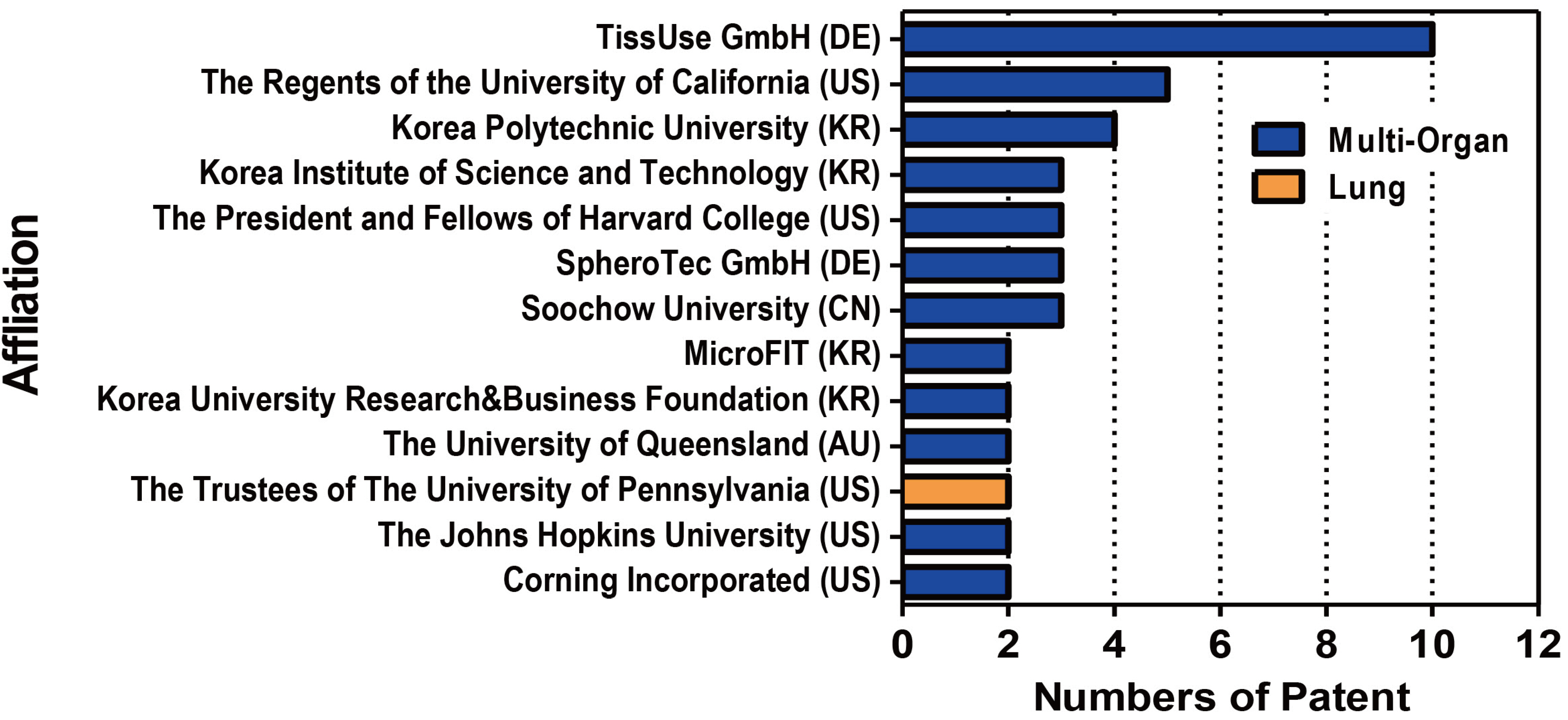
- In vivo animal models are limited in their ability to mimic the extremely complex systems of the human body, and there is increasing disquiet about the ethics of animal research. Many authorities in different geographical areas are considering implementing a ban on animal testing, including testing for cosmetics and pharmaceuticals. Therefore, there is a need for research into systems that can replicate the responses of laboratory animals and simulate environments similar to the human body in a laboratory. An in vitro two-dimensional cell culture model is widely used, because such a system is relatively inexpensive, easy to implement, and can gather considerable amounts of reference data. However, these models lack a real physiological extracellular environment. Recent advances in stem cell biology, tissue engineering, and microfabrication techniques have facilitated the development of various 3D cell culture models. These include multicellular spheroids, organoids, and organs-on-chips, each of which has its own advantages and limitations. Organoids are organ-specific cell clusters created by aggregating cells derived from pluripotent, adult, and cancer stem cells. Patient-derived organoids can be used as models of human disease in a culture dish. Biomimetic organ chips are models that replicate the physiological and mechanical functions of human organs. Many organoids and organ-on-a-chips have been developed for drug screening and testing, so competition for patents between countries is also intensifying. We analyzed the scientific and technological trends underlying these cutting-edge models, which are developed for use as non-animal models for testing safety and efficacy at the nonclinical stages of drug development.
Figure
Reference
-
1. Rawlins MD. 2004; Cutting the cost of drug development? Nat Rev Drug Discov. 3:360–364. DOI: 10.1038/nrd1347. PMID: 15060531.
Article2. Hisha H, Ueno H. 2019; Organoid culture of lingual epithelial cells in a three-dimensional matrix. Methods Mol Biol. 1576:93–99. DOI: 10.1007/7651_2016_3. PMID: 27539458.
Article3. Fentem J, Chamberlain M, Sangster B. 2004; The feasibility of replacing animal testing for assessing consumer safety: a suggested future direction. Altern Lab Anim. 32:617–623. DOI: 10.1177/026119290403200612. PMID: 15757499.
Article4. Heuberger R, Petty M, Huntingford J. 2016; Companion animal owner perceptions, knowledge, and beliefs regarding pain management in end-of-life care. Top Companion Anim Med. 31:152–159. DOI: 10.1053/j.tcam.2017.02.001. PMID: 28317617.
Article5. Jensen J, Hyllner J, Björquist P. 2009; Human embryonic stem cell technologies and drug discovery. J Cell Physiol. 219:513–519. DOI: 10.1002/jcp.21732. PMID: 19277978.
Article6. Caspi O, Itzhaki I, Kehat I, Gepstein A, Arbel G, Huber I, Satin J, Gepstein L. 2009; In vitro electrophysiological drug testing using human embryonic stem cell derived cardiomyocytes. Stem Cells Dev. 18:161–172. DOI: 10.1089/scd.2007.0280. PMID: 18510453.
Article7. Centeno EGZ, Cimarosti H, Bithell A. 2018; 2D versus 3D human induced pluripotent stem cell-derived cultures for neurodegenerative disease modelling. Mol Neurodegener. 13:27. DOI: 10.1186/s13024-018-0258-4. PMID: 29788997. PMCID: PMC5964712.
Article8. Ranga A, Gjorevski N, Lutolf MP. 2014; Drug discovery through stem cell-based organoid models. Adv Drug Deliv Rev. 69-70:19–28. DOI: 10.1016/j.addr.2014.02.006. PMID: 24582599.
Article9. Chen KG, Mallon BS, Park K, Robey PG, McKay RDG, Gottesman MM, Zheng W. 2018; Pluripotent stem cell platforms for drug discovery. Trends Mol Med. 24:805–820. DOI: 10.1016/j.molmed.2018.06.009. PMID: 30006147. PMCID: PMC6117164.
Article10. Lancaster MA, Knoblich JA. 2014; Organogenesis in a dish: modeling development and disease using organoid technologies. Science. 345:1247125. DOI: 10.1126/science.1247125. PMID: 25035496.
Article11. Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, Clevers H. 2009; Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 459:262–265. DOI: 10.1038/nature07935. PMID: 19329995.
Article12. Taguchi A, Kaku Y, Ohmori T, Sharmin S, Ogawa M, Sasaki H, Nishinakamura R. 2014; Redefining the in vivo origin of metanephric nephron progenitors enables generation of complex kidney structures from pluripotent stem cells. Cell Stem Cell. 14:53–67. DOI: 10.1016/j.stem.2013.11.010. PMID: 24332837.13. Xia Y, Nivet E, Sancho-Martinez I, Gallegos T, Suzuki K, Okamura D, Wu MZ, Dubova I, Esteban CR, Montserrat N, Campistol JM, Izpisua Belmonte JC. 2013; Directed differentiation of human pluripotent cells to ureteric bud kidney progenitor-like cells. Nat Cell Biol. 15:1507–1515. DOI: 10.1038/ncb2872. PMID: 24240476.
Article14. Clevers H. 2016; Modeling development and disease with organoids. Cell. 165:1586–1597. DOI: 10.1016/j.cell.2016.05.082. PMID: 27315476.
Article15. Forbester JL, Goulding D, Vallier L, Hannan N, Hale C, Pickard D, Mukhopadhyay S, Dougan G. 2015; Interaction of Salmonella enterica serovar Typhimurium with intestinal organoids derived from human induced pluripotent stem cells. Infect Immun. 83:2926–2934. DOI: 10.1128/IAI.00161-15. PMID: 25964470. PMCID: PMC4468523.
Article16. Leslie JL, Huang S, Opp JS, Nagy MS, Kobayashi M, Young VB, Spence JR. 2015; Persistence and toxin production by Clostridium difficile within human intestinal organoids result in disruption of epithelial paracellular barrier function. Infect Immun. 83:138–145. DOI: 10.1128/IAI.02561-14. PMID: 25312952. PMCID: PMC4288864.
Article17. Spence JR, Mayhew CN, Rankin SA, Kuhar MF, Vallance JE, Tolle K, Hoskins EE, Kalinichenko VV, Wells SI, Zorn AM, Shroyer NF, Wells JM. 2011; Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature. 470:105–109. DOI: 10.1038/nature09691. PMID: 21151107. PMCID: PMC3033971.18. Crespo M, Vilar E, Tsai SY, Chang K, Amin S, Srinivasan T, Zhang T, Pipalia NH, Chen HJ, Witherspoon M, Gordillo M, Xiang JZ, Maxfield FR, Lipkin S, Evans T, Chen S. 2017; Colonic organoids derived from human induced pluripotent stem cells for modeling colorectal cancer and drug testing. Nat Med. 23:878–884. DOI: 10.1038/nm.4355. PMID: 28628110.
Article19. Prior N, Inacio P, Huch M. 2019; Liver organoids: from basic research to therapeutic applications. Gut. 68:2228–2237. DOI: 10.1136/gutjnl-2019-319256. PMID: 31300517. PMCID: PMC6872443.
Article20. Sampaziotis F, de Brito MC, Madrigal P, Bertero A, Saeb-Parsy K, Soares FAC, Schrumpf E, Melum E, Karlsen TH, Bradley JA, Gelson WT, Davies S, Baker A, Kaser A, Alexander GJ, Hannan NRF, Vallier L. 2015; Cholangiocytes derived from human induced pluripotent stem cells for disease modeling and drug validation. Nat Biotechnol. 33:845–852. DOI: 10.1038/nbt.3275. PMID: 26167629. PMCID: PMC4768345.
Article21. Takebe T, Sekine K, Enomura M, Koike H, Kimura M, Ogaeri T, Zhang RR, Ueno Y, Zheng YW, Koike N, Aoyama S, Adachi Y, Taniguchi H. 2013; Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature. 499:481–484. DOI: 10.1038/nature12271. PMID: 23823721.
Article22. Dye BR, Hill DR, Ferguson MA, Tsai YH, Nagy MS, Dyal R, Wells JM, Mayhew CN, Nattiv R, Klein OD, White ES, Deutsch GH, Spence JR. 2015; In vitro generation of human pluripotent stem cell derived lung organoids. Elife. 4:e05098. DOI: 10.7554/eLife.05098. PMID: 25803487. PMCID: PMC4370217.
Article23. Firth AL, Menon T, Parker GS, Qualls SJ, Lewis BM, Ke E, Dargitz CT, Wright R, Khanna A, Gage FH, Verma IM. 2015; Functional gene correction for cystic fibrosis in lung epithelial cells generated from patient iPSCs. Cell Rep. 12:1385–1390. DOI: 10.1016/j.celrep.2015.07.062. PMID: 26299960. PMCID: PMC4559351.
Article24. Garcez PP, Loiola EC, Madeiro da Costa R, Higa LM, Trindade P, Delvecchio R, Nascimento JM, Brindeiro R, Tanuri A, Rehen SK. 2016; Zika virus impairs growth in human neurospheres and brain organoids. Science. 352:816–818. DOI: 10.1126/science.aaf6116. PMID: 27064148.
Article25. Hohwieler M, Illing A, Hermann PC, Mayer T, Stockmann M, Perkhofer L, Eiseler T, Antony JS, Müller M, Renz S, Kuo CC, Lin Q, Sendler M, Breunig M, Kleiderman SM, Lechel A, Zenker M, Leichsenring M, Rosendahl J, Zenke M, et al. 2017; Human pluripotent stem cell-derived acinar/ductal organoids generate human pancreas upon orthotopic transplantation and allow disease modelling. Gut. 66:473–486. DOI: 10.1136/gutjnl-2016-312423. PMID: 27633923. PMCID: PMC5534761.
Article26. Huang L, Holtzinger A, Jagan I, BeGora M, Lohse I, Ngai N, Nostro C, Wang R, Muthuswamy LB, Crawford HC, Arrowsmith C, Kalloger SE, Renouf DJ, Connor AA, Cleary S, Schaeffer DF, Roehrl M, Tsao MS, Gallinger S, Keller G, et al. 2015; Ductal pancreatic cancer modeling and drug screening using human pluripotent stem cell- and patient-derived tumor organoids. Nat Med. 21:1364–1371. DOI: 10.1038/nm.3973. PMID: 26501191. PMCID: PMC4753163.
Article27. Mariani J, Coppola G, Zhang P, Abyzov A, Provini L, Tomasini L, Amenduni M, Szekely A, Palejev D, Wilson M, Gerstein M, Grigorenko EL, Chawarska K, Pelphrey KA, Howe JR, Vaccarino FM. 2015; FOXG1-dependent dysregulation of GABA/glutamate neuron differentiation in autism spectrum disorders. Cell. 162:375–390. DOI: 10.1016/j.cell.2015.06.034. PMID: 26186191. PMCID: PMC4519016.
Article28. Qian X, Nguyen HN, Jacob F, Song H, Ming GL. 2017; Using brain organoids to understand Zika virus-induced microcephaly. Development. 144:952–957. DOI: 10.1242/dev.140707. PMID: 28292840. PMCID: PMC5358105.
Article29. Drost J, Clevers H. 2017; Translational applications of adult stem cell-derived organoids. Development. 144:968–975. DOI: 10.1242/dev.140566. PMID: 28292843.
Article30. Hisha H, Tanaka T, Kanno S, Tokuyama Y, Komai Y, Ohe S, Yanai H, Omachi T, Ueno H. 2013; Establishment of a novel lingual organoid culture system: generation of organoids having mature keratinized epithelium from adult epithelial stem cells. Sci Rep. 3:3224. DOI: 10.1038/srep03224. PMID: 24232854. PMCID: PMC3828633.
Article31. Barker N, Huch M, Kujala P, van de Wetering M, Snippert HJ, van Es JH, Sato T, Stange DE, Begthel H, van den Born M, Danenberg E, van den Brink S, Korving J, Abo A, Peters PJ, Wright N, Poulsom R, Clevers H. 2010; Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell. 6:25–36. DOI: 10.1016/j.stem.2009.11.013. PMID: 20085740.
Article32. Bartfeld S, Bayram T, van de Wetering M, Huch M, Begthel H, Kujala P, Vries R, Peters PJ, Clevers H. 2015; In vitro expansion of human gastric epithelial stem cells and their responses to bacterial infection. Gastroenterology. 148:126–136.e6. DOI: 10.1053/j.gastro.2014.09.042. PMID: 25307862. PMCID: PMC4274199.33. Drost J, van Jaarsveld RH, Ponsioen B, Zimberlin C, van Boxtel R, Buijs A, Sachs N, Overmeer RM, Offerhaus GJ, Begthel H, Korving J, van de Wetering M, Schwank G, Logtenberg M, Cuppen E, Snippert HJ, Medema JP, Kops GJ, Clevers H. 2015; Sequential cancer mutations in cultured human intestinal stem cells. Nature. 521:43–47. DOI: 10.1038/nature14415. PMID: 25924068.
Article34. Fair KL, Colquhoun J, Hannan NRF. 2018; Intestinal organoids for modelling intestinal development and disease. Philos Trans R Soc Lond B Biol Sci. 373:20170217. DOI: 10.1098/rstb.2017.0217. PMID: 29786552. PMCID: PMC5974440.
Article35. Xu H, Lyu X, Yi M, Zhao W, Song Y, Wu K. 2018; Organoid technology and applications in cancer research. J Hematol Oncol. 11:116. DOI: 10.1186/s13045-018-0662-9. PMID: 30219074. PMCID: PMC6139148.
Article36. Papafragkou E, Hewitt J, Park GW, Greening G, Vinjé J. 2013; Challenges of culturing human norovirus in three-dimensional organoid intestinal cell culture models. PLoS One. 8:e63485. DOI: 10.1371/journal.pone.0063485. PMID: 23755105. PMCID: PMC3670855.
Article37. Schwank G, Koo BK, Sasselli V, Dekkers JF, Heo I, Demircan T, Sasaki N, Boymans S, Cuppen E, van der Ent CK, Nieuwenhuis EE, Beekman JM, Clevers H. 2013; Functional repair of CFTR by CRISPR/Cas9 in intestinal stem cell organoids of cystic fibrosis patients. Cell Stem Cell. 13:653–658. DOI: 10.1016/j.stem.2013.11.002. PMID: 24315439.
Article38. Sato T, Stange DE, Ferrante M, Vries RG, Van Es JH, Van den Brink S, Van Houdt WJ, Pronk A, Van Gorp J, Siersema PD, Clevers H. 2011; Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett's epithelium. Gastroenterology. 141:1762–1772. DOI: 10.1053/j.gastro.2011.07.050. PMID: 21889923.
Article39. Dutta D, Heo I, Clevers H. 2017; Disease modeling in stem cell-derived 3D organoid systems. Trends Mol Med. 23:393–410. DOI: 10.1016/j.molmed.2017.02.007. PMID: 28341301.
Article40. Huch M, Gehart H, van Boxtel R, Hamer K, Blokzijl F, Verstegen MM, Ellis E, van Wenum M, Fuchs SA, de Ligt J, van de Wetering M, Sasaki N, Boers SJ, Kemperman H, de Jonge J, Ijzermans JN, Nieuwenhuis EE, Hoekstra R, Strom S, Vries RR, et al. 2015; Long-term culture of genome-stable bipotent stem cells from adult human liver. Cell. 160:299–312. DOI: 10.1016/j.cell.2014.11.050. PMID: 25533785. PMCID: PMC4313365.
Article41. Guan Y, Xu D, Garfin PM, Ehmer U, Hurwitz M, Enns G, Michie S, Wu M, Zheng M, Nishimura T, Sage J, Peltz G. 2017; Human hepatic organoids for the analysis of human genetic diseases. JCI Insight. 2:e94954. DOI: 10.1172/jci.insight.94954. PMID: 28878125. PMCID: PMC5621886.
Article42. Fiorotto R, Amenduni M, Mariotti V, Fabris L, Spirli C, Strazzabosco M. 2018; Src kinase inhibition reduces inflammatory and cytoskeletal changes in ΔF508 human cholangiocytes and improves cystic fibrosis transmembrane conductance regulator correctors efficacy. Hepatology. 67:972–988. DOI: 10.1002/hep.29400. PMID: 28836688. PMCID: PMC5783790.
Article43. Broutier L, Mastrogiovanni G, Verstegen MM, Francies HE, Gavarró LM, Bradshaw CR, Allen GE, Arnes-Benito R, Sidorova O, Gaspersz MP, Georgakopoulos N, Koo BK, Dietmann S, Davies SE, Praseedom RK, Lieshout R, IJzermans JNM, Wigmore SJ, Saeb-Parsy K, Garnett MJ, et al. 2017; Human primary liver cancer-derived organoid cultures for disease modeling and drug screening. Nat Med. 23:1424–1435. DOI: 10.1038/nm.4438. PMID: 29131160. PMCID: PMC5722201.
Article44. Sun L, Wang Y, Cen J, Ma X, Cui L, Qiu Z, Zhang Z, Li H, Yang RZ, Wang C, Chen X, Wang L, Ye Y, Zhang H, Pan G, Kang JS, Ji Y, Zheng YW, Zheng S, Hui L. 2019; Modelling liver cancer initiation with organoids derived from directly reprogrammed human hepatocytes. Nat Cell Biol. 21:1015–1026. DOI: 10.1038/s41556-019-0359-5. PMID: 31332348.
Article45. Kim M, Mun H, Sung CO, Cho EJ, Jeon HJ, Chun SM, Jung DJ, Shin TH, Jeong GS, Kim DK, Choi EK, Jeong SY, Taylor AM, Jain S, Meyerson M, Jang SJ. 2019; Patient-derived lung cancer organoids as in vitro cancer models for therapeutic screening. Nat Commun. 10:3991. DOI: 10.1038/s41467-019-11867-6. PMID: 31488816. PMCID: PMC6728380.
Article46. Lancaster MA, Renner M, Martin CA, Wenzel D, Bicknell LS, Hurles ME, Homfray T, Penninger JM, Jackson AP, Knoblich JA. 2013; Cerebral organoids model human brain development and microcephaly. Nature. 501:373–379. DOI: 10.1038/nature12517. PMID: 23995685. PMCID: PMC3817409.
Article47. Wells MF, Salick MR, Wiskow O, Ho DJ, Worringer KA, Ihry RJ, Kommineni S, Bilican B, Klim JR, Hill EJ, Kane LT, Ye C, Kaykas A, Eggan K. 2016; Genetic ablation of AXL does not protect human neural progenitor cells and cerebral organoids from Zika virus infection. Cell Stem Cell. 19:703–708. DOI: 10.1016/j.stem.2016.11.011. PMID: 27912091.
Article48. Hubert CG, Rivera M, Spangler LC, Wu Q, Mack SC, Prager BC, Couce M, McLendon RE, Sloan AE, Rich JN. 2016; A three-dimensional organoid culture system derived from human glioblastomas recapitulates the hypoxic gradients and cancer stem cell heterogeneity of tumors found in vivo. Cancer Res. 76:2465–2477. DOI: 10.1158/0008-5472.CAN-15-2402. PMID: 26896279. PMCID: PMC4873351.49. Ogawa J, Pao GM, Shokhirev MN, Verma IM. 2018; Glioblastoma model using human cerebral organoids. Cell Rep. 23:1220–1229. DOI: 10.1016/j.celrep.2018.03.105. PMID: 29694897. PMCID: PMC6892608.
Article50. Dotti I, Mora-Buch R, Ferrer-Picón E, Planell N, Jung P, Masamunt MC, Leal RF, Martín de Carpi J, Llach J, Ordás I, Batlle E, Panés J, Salas A. 2017; Alterations in the epithelial stem cell compartment could contribute to permanent changes in the mucosa of patients with ulcerative colitis. Gut. 66:2069–2079. DOI: 10.1136/gutjnl-2016-312609. PMID: 27803115. PMCID: PMC5749340.
Article51. Quaranta M, Sébert M, Bonnet D, Kirzin S, Portier G, Duffas JP, Chabot S, Lluel P, Allart S, Ferrand A, Alric L, Racaud-Sultan C, Mas E, Deraison C, Vergnolle N. d'Aldebert E. 2020; Characterization of human colon organoids from inflammatory bowel disease patients. Front Cell Dev Biol. 8:363. DOI: 10.3389/fcell.2020.00363. PMID: 32582690. PMCID: PMC7287042.
Article52. Dekkers JF, Berkers G, Kruisselbrink E, Vonk A, de Jonge HR, Janssens HM, Bronsveld I, van de Graaf EA, Nieuwenhuis EE, Houwen RH, Vleggaar FP, Escher JC, de Rijke YB, Majoor CJ, Heijerman HG, de Winter-de Groot KM, Clevers H, van der Ent CK, Beekman JM. 2016; Characterizing responses to CFTR-modulating drugs using rectal organoids derived from subjects with cystic fibrosis. Sci Transl Med. 8:344ra84. DOI: 10.1126/scitranslmed.aad8278. PMID: 27334259.
Article53. Finkbeiner SR, Zeng XL, Utama B, Atmar RL, Shroyer NF, Estes MK. 2012; Stem cell-derived human intestinal organoids as an infection model for rotaviruses. mBio. 3:e00159–e00112. DOI: 10.1128/mBio.00159-12. PMID: 22761392. PMCID: PMC3398537.
Article54. de Witte CJ, Espejo Valle-Inclan J, Hami N, Lõhmussaar K, Kopper O, Vreuls CPH, Jonges GN, van Diest P, Nguyen L, Clevers H, Kloosterman WP, Cuppen E, Snippert HJG, Zweemer RP, Witteveen PO, Stelloo E. 2020; Patient-derived ovarian cancer organoids mimic clinical response and exhibit heterogeneous inter- and intrapatient drug responses. Cell Rep. 31:107762. DOI: 10.1016/j.celrep.2020.107762. PMID: 32553164.
Article55. Huang KC, Wang ML, Chen SJ, Kuo JC, Wang WJ, Nhi Nguyen PN, Wahlin KJ, Lu JF, Tran AA, Shi M, Chien Y, Yarmishyn AA, Tsai PH, Yang TC, Jane WN, Chang CC, Peng CH, Schlaeger TM, Chiou SH. 2019; Morphological and molecular defects in human three-dimensional retinal organoid model of X-linked juvenile retinoschisis. Stem Cell Reports. 13:906–923. DOI: 10.1016/j.stemcr.2019.09.010. PMID: 31668851. PMCID: PMC6895767.
Article56. Takasato M, Er PX, Chiu HS, Maier B, Baillie GJ, Ferguson C, Parton RG, Wolvetang EJ, Roost MS, Chuva de Sousa Lopes SM, Little MH. 2015; Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature. 526:564–568. DOI: 10.1038/nature15695. PMID: 26444236.
Article57. Freedman BS, Brooks CR, Lam AQ, Fu H, Morizane R, Agrawal V, Saad AF, Li MK, Hughes MR, Werff RV, Peters DT, Lu J, Baccei A, Siedlecki AM, Valerius MT, Musunuru K, McNagny KM, Steinman TI, Zhou J, Lerou PH, et al. 2015; Modelling kidney disease with CRISPR-mutant kidney organoids derived from human pluripotent epiblast spheroids. Nat Commun. 6:8715. DOI: 10.1038/ncomms9715. PMID: 26493500. PMCID: PMC4620584.
Article58. Trisno SL, Philo KED, McCracken KW, Catá EM, Ruiz-Torres S, Rankin SA, Han L, Nasr T, Chaturvedi P, Rothenberg ME, Mandegar MA, Wells SI, Zorn AM, Wells JM. 2018; Esophageal organoids from human pluripotent stem cells delineate Sox2 functions during esophageal specification. Cell Stem Cell. 23:501–515.e7. DOI: 10.1016/j.stem.2018.08.008. PMID: 30244869. PMCID: PMC6225525.
Article59. Nugraha B, Buono MF, von Boehmer L, Hoerstrup SP, Emmert MY. 2019; Human cardiac organoids for disease modeling. Clin Pharmacol Ther. 105:79–85. DOI: 10.1002/cpt.1286. PMID: 30415499.
Article60. Sachs N, de Ligt J, Kopper O, Gogola E, Bounova G, Weeber F, Balgobind AV, Wind K, Gracanin A, Begthel H, Korving J, van Boxtel R, Duarte AA, Lelieveld D, van Hoeck A, Ernst RF, Blokzijl F, Nijman IJ, Hoogstraat M, van de Ven M, et al. 2018; A living biobank of breast cancer organoids captures disease heterogeneity. Cell. 172:373–386.e10. DOI: 10.1016/j.cell.2017.11.010. PMID: 29224780.
Article61. Bartfeld S, Clevers H. 2015; Organoids as model for infectious diseases: culture of human and murine stomach organoids and microinjection of Helicobacter pylori. J Vis Exp. (105):53359. DOI: 10.3791/53359. PMID: 26650279. PMCID: PMC4692704.
Article62. Shik Mun K, Arora K, Huang Y, Yang F, Yarlagadda S, Ramananda Y, Abu-El-Haija M, Palermo JJ, Appakalai BN, Nathan JD, Naren AP. 2019; Patient-derived pancreas-on-a-chip to model cystic fibrosis-related disorders. Nat Commun. 10:3124. DOI: 10.1038/s41467-019-11178-w. PMID: 31311920. PMCID: PMC6635497.
Article63. Boj SF, Hwang CI, Baker LA, Chio II, Engle DD, Corbo V, Jager M, Ponz-Sarvise M, Tiriac H, Spector MS, Gracanin A, Oni T, Yu KH, van Boxtel R, Huch M, Rivera KD, Wilson JP, Feigin ME, Öhlund D, Handly-Santana A, et al. 2015; Organoid models of human and mouse ductal pancreatic cancer. Cell. 160:324–338. DOI: 10.1016/j.cell.2014.12.021. PMID: 25557080.
Article64. Tuveson D, Clevers H. 2019; Cancer modeling meets human organoid technology. Science. 364:952–955. DOI: 10.1126/science.aaw6985. PMID: 31171691.
Article65. Byrne AT, Alférez DG, Amant F, Annibali D, Arribas J, Biankin AV, Bruna A, Budinská E, Caldas C, Chang DK, Clarke RB, Clevers H, Coukos G, Dangles-Marie V, Eckhardt SG, Gonzalez-Suarez E, Hermans E, Hidalgo M, Jarzabek MA, de Jong S, et al. 2017; Interrogating open issues in cancer precision medicine with patient-derived xenografts. Nat Rev Cancer. 17:254–268. DOI: 10.1038/nrc.2016.140. PMID: 28104906.
Article66. Drost J, Clevers H. 2018; Organoids in cancer research. Nat Rev Cancer. 18:407–418. DOI: 10.1038/s41568-018-0007-6. PMID: 29692415.
Article67. van de Wetering M, Francies HE, Francis JM, Bounova G, Iorio F, Pronk A, van Houdt W, van Gorp J, Taylor-Weiner A, Kester L, McLaren-Douglas A, Blokker J, Jaksani S, Bartfeld S, Volckman R, van Sluis P, Li VS, Seepo S, Sekhar Pedamallu C, Cibulskis K, et al. 2015; Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell. 161:933–945. DOI: 10.1016/j.cell.2015.03.053. PMID: 25957691. PMCID: PMC6428276.
Article68. Baker LA, Tiriac H, Clevers H, Tuveson DA. 2016; Modeling pancreatic cancer with organoids. Trends Cancer. 2:176–190. DOI: 10.1016/j.trecan.2016.03.004. PMID: 27135056. PMCID: PMC4847151.
Article69. Kuo CJ, Curtis C. 2018; Organoids reveal cancer dynamics. Nature. 556:441–442. DOI: 10.1038/d41586-018-03841-x. PMID: 29686366.
Article70. Ishida S. 2018; Organs-on-a-chip: current applications and consideration points for in vitro ADME-Tox studies. Drug Metab Pharmacokinet. 33:49–54. DOI: 10.1016/j.dmpk.2018.01.003. PMID: 29398302.71. Kimura H, Sakai Y, Fujii T. 2018; Organ/body-on-a-chip based on microfluidic technology for drug discovery. Drug Metab Pharmacokinet. 33:43–48. DOI: 10.1016/j.dmpk.2017.11.003. PMID: 29175062.
Article72. Chi CW, Ahmed AR, Dereli-Korkut Z, Wang S. 2016; Microfluidic cell chips for high-throughput drug screening. Bioanalysis. 8:921–937. DOI: 10.4155/bio-2016-0028. PMID: 27071838. PMCID: PMC4870726.
Article73. Livingston CA, Fabre KM, Tagle DA. 2016; Facilitating the commercialization and use of organ platforms generated by the microphysiological systems (Tissue Chip) program through public-private partnerships. Comput Struct Biotechnol J. 14:207–210. DOI: 10.1016/j.csbj.2016.04.003. PMID: 27904714. PMCID: PMC5122750.
Article74. Low LA, Tagle DA. 2016; Tissue chips to aid drug development and modeling for rare diseases. Expert Opin Orphan Drugs. 4:1113–1121. DOI: 10.1080/21678707.2016.1244479. PMID: 28626620. PMCID: PMC5472383.
Article75. Alcendor DJ, Block FE 3rd, Cliffel DE, Daniels JS, Ellacott KL, Goodwin CR, Hofmeister LH, Li D, Markov DA, May JC, McCawley LJ, McLaughlin B, McLean JA, Niswender KD, Pensabene V, Seale KT, Sherrod SD, Sung HJ, Tabb DL, Webb DJ, et al. 2013; Neurovascular unit on a chip: implications for translational applications. Stem Cell Res Ther. 4(Suppl 1):S18. DOI: 10.1186/scrt379. PMID: 24564885. PMCID: PMC4029462.
Article76. Mathur A, Loskill P, Shao K, Huebsch N, Hong S, Marcus SG, Marks N, Mandegar M, Conklin BR, Lee LP, Healy KE. 2015; Human iPSC-based cardiac microphysiological system for drug screening applications. Sci Rep. 5:8883. DOI: 10.1038/srep08883. PMID: 25748532. PMCID: PMC4352848.
Article77. Agarwal A, Goss JA, Cho A, McCain ML, Parker KK. 2013; Microfluidic heart on a chip for higher throughput pharmacological studies. Lab Chip. 13:3599–3608. DOI: 10.1039/c3lc50350j. PMID: 23807141. PMCID: PMC3786400.
Article78. Nesmith AP, Agarwal A, McCain ML, Parker KK. 2014; Human airway musculature on a chip: an in vitro model of allergic asthmatic bronchoconstriction and bronchodilation. Lab Chip. 14:3925–3936. DOI: 10.1039/C4LC00688G. PMID: 25093641.79. Vernetti LA, Senutovitch N, Boltz R, DeBiasio R, Shun TY, Gough A, Taylor DL. 2016; A human liver microphysiology platform for investigating physiology, drug safety, and disease models. Exp Biol Med (Maywood). 241:101–114. DOI: 10.1177/1535370215592121. PMID: 26202373. PMCID: PMC4723301.
Article80. Song H, Peng S, Chen T, Zhang B, Wang Z. Cell cytotoxicity analysis of adherent and suspended cancer cells using an integrated microfluidic device. presented at: 2011 4th International Conference on Biomedical Engineering and Informatics (BMEI). 2011 Oct 15-17; Shanghai, China. p. 1051–1054. DOI: 10.1109/BMEI.2011.6098477.81. Male KB, Lachance B, Hrapovic S, Sunahara G, Luong JH. 2008; Assessment of cytotoxicity of quantum dots and gold nanoparticles using cell-based impedance spectroscopy. Anal Chem. 80:5487–5493. DOI: 10.1021/ac8004555. PMID: 18553941.
Article82. Zheng GX, Li YJ, Qi LL, Liu XM, Wang H, Yu SP, Wang YH. 2014; Marine phytoplankton motility sensor integrated into a microfluidic chip for high-throughput pollutant toxicity assessment. Mar Pollut Bull. 84:147–154. DOI: 10.1016/j.marpolbul.2014.05.019. PMID: 24882443.
Article83. Kloss D, Fischer M, Rothermel A, Simon JC, Robitzki AA. 2008; Drug testing on 3D in vitro tissues trapped on a microcavity chip. Lab Chip. 8:879–884. DOI: 10.1039/b800394g. PMID: 18497906.
Article84. Huh D, Leslie DC, Matthews BD, Fraser JP, Jurek S, Hamilton GA, Thorneloe KS, McAlexander MA, Ingber DE. 2012; A human disease model of drug toxicity-induced pulmonary edema in a lung-on-a-chip microdevice. Sci Transl Med. 4:159ra147. DOI: 10.1126/scitranslmed.3004249. PMID: 23136042.
Article85. Fattahi P, Yang G, Kim G, Abidian MR. 2014; A review of organic and inorganic biomaterials for neural interfaces. Adv Mater. 26:1846–1885. DOI: 10.1002/adma.201304496. PMID: 24677434. PMCID: PMC4373558.
Article86. Hou C, Duan Y, Zhang Q, Wang H, Li Y. 2012; Bio-applicable and electroactive near-infrared laser-triggered self-healing hydrogels based on graphene networks. J Mater Chem. 22:14991–14996. DOI: 10.1039/c2jm32255b.
Article87. Arndt S, Seebach J, Psathaki K, Galla HJ, Wegener J. 2004; Bioelectrical impedance assay to monitor changes in cell shape during apoptosis. Biosens Bioelectron. 19:583–594. DOI: 10.1016/S0956-5663(03)00269-0. PMID: 14683642.
Article88. Rothbauer M, Zirath H, Ertl P. 2018; Recent advances in microfluidic technologies for cell-to-cell interaction studies. Lab Chip. 18:249–270. DOI: 10.1039/C7LC00815E. PMID: 29143053.
Article89. Kim R, Nam Y. 2015; Electrochemical layer-by-layer approach to fabricate mechanically stable platinum black microelectrodes using a mussel-inspired polydopamine adhesive. J Neural Eng. 12:026010. DOI: 10.1088/1741-2560/12/2/026010. PMID: 25738544.
Article90. Mohammadniaei M, Yoon J, Lee T, Bharate BG, Jo J, Lee D, Choi JW. 2018; Electrochemical biosensor composed of silver ion-mediated dsDNA on Au-encapsulated Bi2 Se3 nanoparticles for the detection of H2O2 released from breast cancer cells. Small. 14:e1703970. DOI: 10.1002/smll.201703970. PMID: 29573539.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical application of human embryonic stem cells
- Biofabricated 3D Intestinal Models as an Alternative to AnimalBased Approaches for Drug Toxicity Assays
- The Role of Large Animal Studies in Cardiac Regenerative Therapy Concise Review of Translational Stem Cell Research
- Current Challenges Associated with the Use of Human Induced Pluripotent Stem Cell-Derived Organoids in Regenerative Medicine
- Latest Trends in Non-Clinical and Clinical Research on Mesenchymal Stem Cell Therapy for Tendon Regeneration