Korean J Ophthalmol.
2020 Apr;34(2):97-105. 10.3341/kjo.2019.0124.
Effect of Minoxidil on Trabecular Outflow via the Paracellular Pathway
- Affiliations
-
- 1Cheil Eye Hospital, Daegu, Korea.
- 2Department of Ophthalmology, Daegu Catholic University School of Medicine, Daegu, Korea.
- KMID: 2507398
- DOI: http://doi.org/10.3341/kjo.2019.0124
Abstract
- Purpose
To investigate the pathway and effects of minoxidil on trabecular outflow in cultured human trabecular meshwork (TM) cells.
Methods
After exposing primarily cultured TM cells to 0, 10, 50, or 100 µM minoxidil sulfate (MS), trabecular outflow was assessed by measuring TM cell monolayer permeability to carboxyfluorescein and transepithelial electrical resistance. To assess the pathway of permeability changes, caveolin-1, occludin, and claudin-5 levels were measured via western blot. Generation of reactive oxygen species (ROS) was measured using the dichlorofluorescein diacetate assay. To assess the involvement of nitric oxide (NO) in minoxidil-induced permeability increase, the degrees of endothelial nitric oxide synthase mRNA expression and NO production were measured with reverse transcription polymerase chain reaction and Griess assays, respectively. Permeability was also measured with co-exposure to 50 µM N-acetyl cysteine.
Results
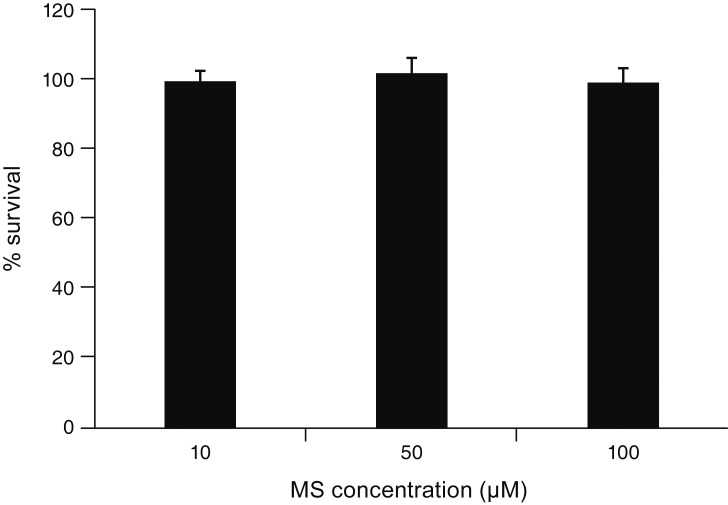
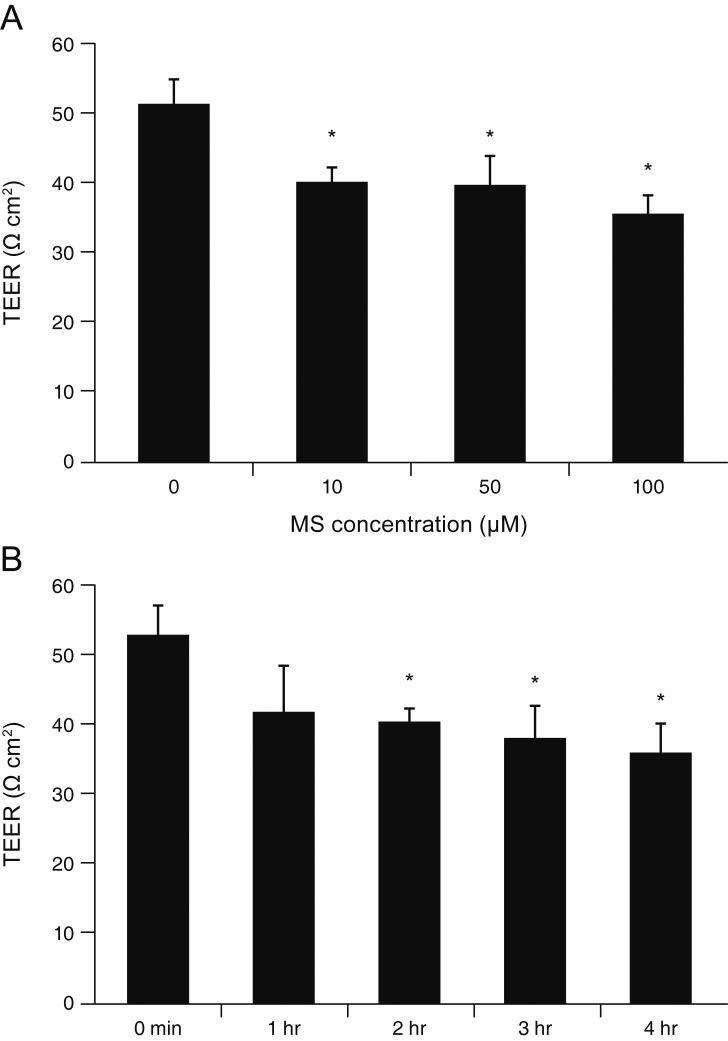
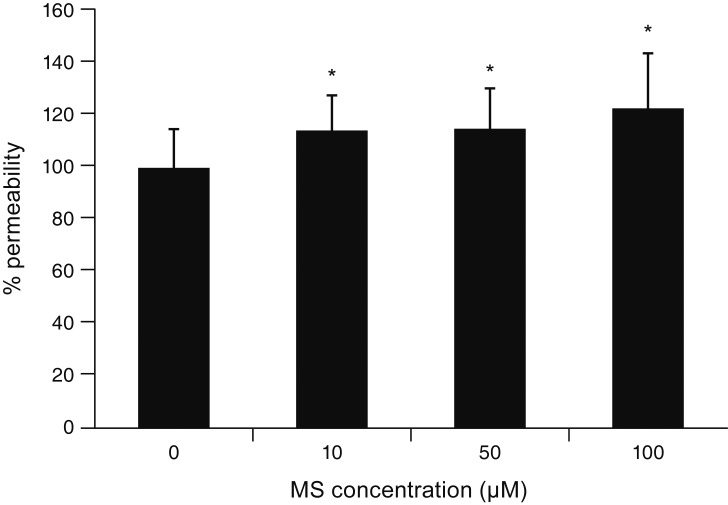
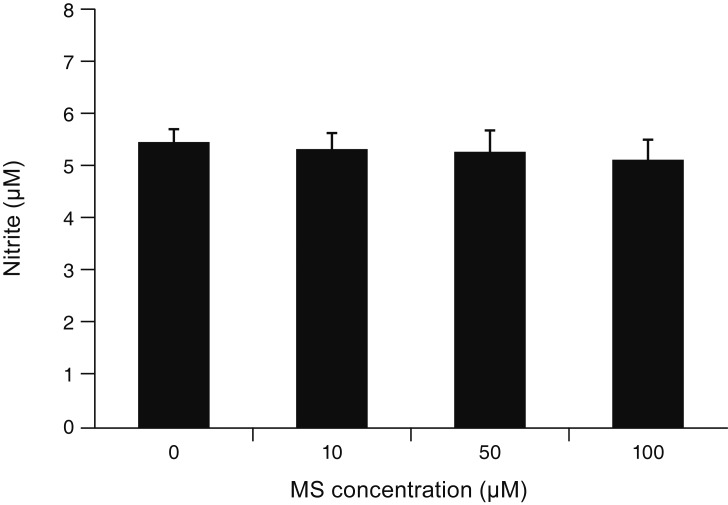
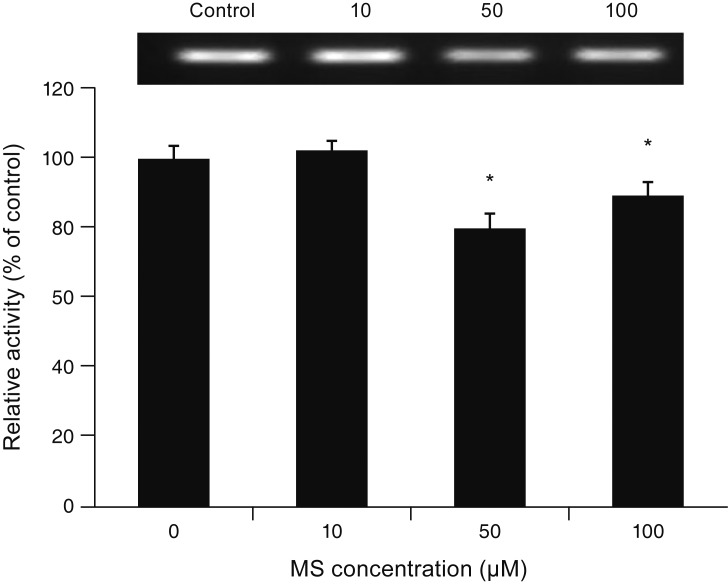
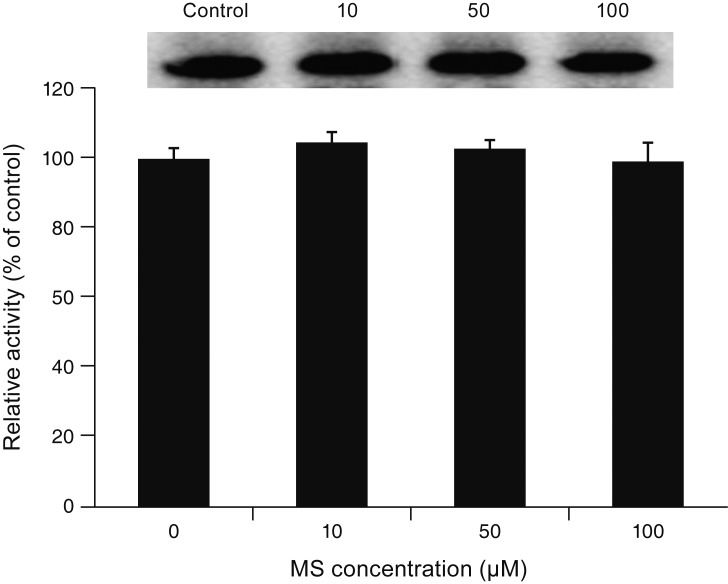
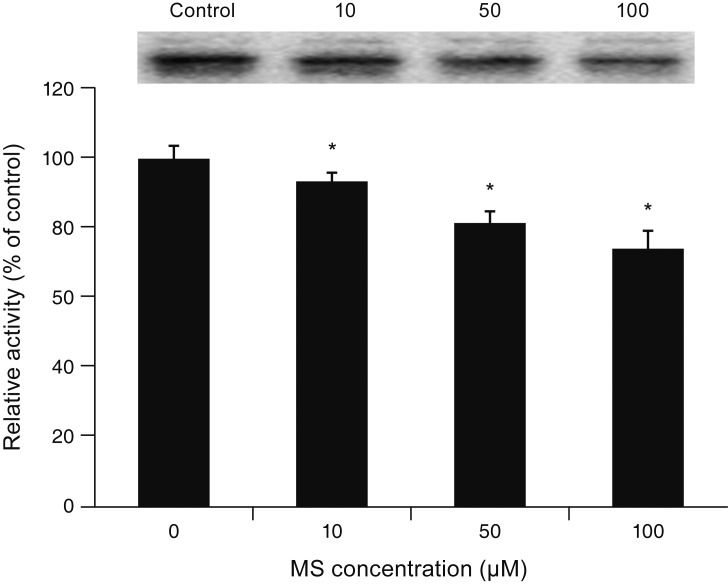
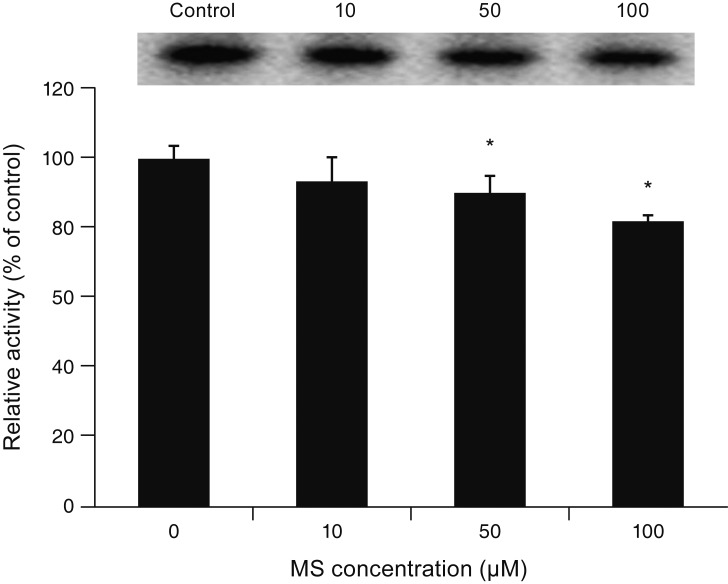
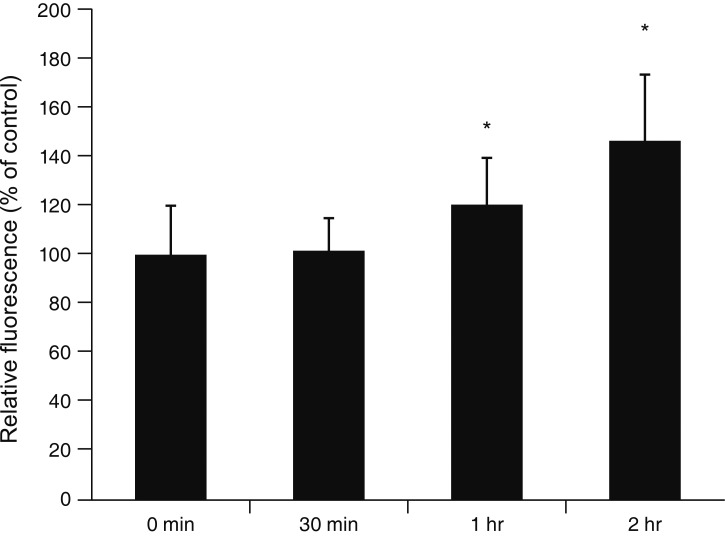
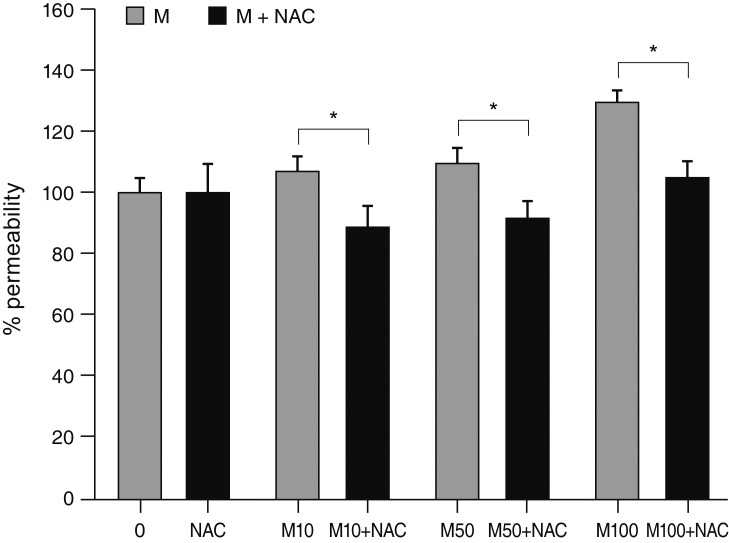
MS significantly increased TM cell monolayer permeability (p < 0.05) and decreased transepithelial electrical resistance (p < 0.05). MS decreased the degree of endothelial nitric oxide synthase mRNA expression but did not affect NO production. MS decreased occludin and claudin-5 levels but did not affect caveolin-1 level. MS at 100 µM increased the generation of ROS, and MS-induced permeability increase was attenuated after co-exposure to 50 µM N-acetyl cysteine.
Conclusions
Minoxidil may preferentially increase trabecular permeability via a paracellular pathway by downregulation of tight junction proteins. This minoxidil-induced permeability through the TM may be mediated by generation of ROS.
Figure
Reference
-
1. Alvarado J, Murphy C, Juster R. Trabecular meshwork cellularity in primary open-angle glaucoma and nonglaucomatous normals. Ophthalmology. 1984; 91:564–579. PMID: 6462622.
Article2. Rohen JW, Lutjen-Drecoll E, Flugel C, et al. Ultrastructure of the trabecular meshwork in untreated cases of primary open-angle glaucoma (POAG). Exp Eye Res. 1993; 56:683–692. PMID: 8595810.3. Kopczynski CC, Epstein DL. Emerging trabecular outflow drugs. J Ocul Pharmacol Ther. 2014; 30:85–87. PMID: 24304197.
Article4. Ashcroft FM, Gribble FM. New windows on the mechanism of action of K(ATP) channel openers. Trends Pharmacol Sci. 2000; 21:439–445. PMID: 11121575.5. Sica DA. Minoxidil: an underused vasodilator for resistant or severe hypertension. J Clin Hypertens (Greenwich). 2004; 6:283–287. PMID: 15133413.
Article6. Nathanson JA. Nitrovasodilators as a new class of ocular hypotensive agents. J Pharmacol Exp Ther. 1992; 260:956–965. PMID: 1532035.7. Ningaraj NS, Rao MK, Black KL. Adenosine 5′-triphosphate-sensitive potassium channel-mediated blood-brain tumor barrier permeability increase in a rat brain tumor model. Cancer Res. 2003; 63:8899–8911. PMID: 14695207.8. Gu YT, Xue YX, Wang YF, et al. Role of ROS/RhoA/PI3K/PKB signaling in NS1619-mediated blood-tumor barrier permeability increase. J Mol Neurosci. 2012; 48:302–312. PMID: 22581438.
Article9. Gu YT, Xue YX, Wang YF, et al. Minoxidil sulfate induced the increase in blood-brain tumor barrier permeability through ROS/RhoA/PI3K/PKB signaling pathway. Neuropharmacology. 2013; 75:407–415. PMID: 23973310.
Article10. Komarova Y, Malik AB. Regulation of endothelial permeability via paracellular and transcellular transport pathways. Annu Rev Physiol. 2010; 72:463–493. PMID: 20148685.
Article11. Wolburg H, Lippoldt A. Tight junctions of the blood-brain barrier: development, composition and regulation. Vascul Pharmacol. 2002; 38:323–337. PMID: 12529927.12. Quest AF, Gutierrez-Pajares JL, Torres VA. Caveolin-1: an ambiguous partner in cell signalling and cancer. J Cell Mol Med. 2008; 12:1130–1150. PMID: 18400052.
Article13. Sun SW, Zu XY, Tuo QH, et al. Caveolae and caveolin-1 mediate endocytosis and transcytosis of oxidized low density lipoprotein in endothelial cells. Acta Pharmacol Sin. 2010; 31:1336–1342. PMID: 20835266.
Article14. Surgucheva I, Surguchov A. Expression of caveolin in trabecular meshwork cells and its possible implication in pathogenesis of primary open angle glaucoma. Mol Vis. 2011; 17:2878–2888. PMID: 22128235.15. Gu YT, Xue YX, Zhang H, et al. Adenosine 5′-triphosphate-sensitive potassium channel activator induces the up-regulation of caveolin-1 expression in a rat brain tumor model. Cell Mol Neurobiol. 2011; 31:629–634. PMID: 21331626.
Article16. Nag S, Venugopalan R, Stewart DJ. Increased caveolin-1 expression precedes decreased expression of occludin and claudin-5 during blood-brain barrier breakdown. Acta Neuropathol. 2007; 114:459–469. PMID: 17687559.
Article17. Stamer WD, Clark AF. The many faces of the trabecular meshwork cell. Exp Eye Res. 2017; 158:112–123. PMID: 27443500.
Article18. Gipson IK, Anderson RA. Actin filaments in cells of human trabecular meshwork and Schlemm's canal. Invest Ophthalmol Vis Sci. 1979; 18:547–561. PMID: 571861.19. Lepple-Wienhues A, Rauch R, Clark AF, et al. Electrophysiological properties of cultured human trabecular meshwork cells. Exp Eye Res. 1994; 59:305–311. PMID: 7821375.
Article20. Wiederholt M, Bielka S, Schweig F, et al. Regulation of outflow rate and resistance in the perfused anterior segment of the bovine eye. Exp Eye Res. 1995; 61:223–234. PMID: 7556486.
Article21. Wiederholt M, Thieme H, Stumpff F. The regulation of trabecular meshwork and ciliary muscle contractility. Prog Retin Eye Res. 2000; 19:271–295. PMID: 10749378.
Article22. Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983; 65:55–63. PMID: 6606682.
Article23. Freimoser FM, Jakob CA, Aebi M, Tuor U. The MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay is a fast and reliable method for colorimetric determination of fungal cell densities. Appl Environ Microbiol. 1999; 65:3727–3729. PMID: 10427074.
Article24. Araie M. Carboxyfluorescein. A dye for evaluating the corneal endothelial barrier function in vivo. Exp Eye Res. 1986; 42:141–150. PMID: 3699104.
Article25. Grimes PA. Carboxyfluorescein transfer across the blood-retinal barrier evaluated by quantitative fluorescence microscopy: comparison with fluorescein. Exp Eye Res. 1988; 46:769–783. PMID: 3384022.
Article26. Nakagawa S, Usui T, Yokoo S, et al. Toxicity evaluation of antiglaucoma drugs using stratified human cultivated corneal epithelial sheets. Invest Ophthalmol Vis Sci. 2012; 53:5154–5160. PMID: 22695966.
Article27. Lei Y, Stamer WD, Wu J, Sun X. Oxidative stress impact on barrier function of porcine angular aqueous plexus cell monolayers. Invest Ophthalmol Vis Sci. 2013; 54:4827–4835. PMID: 23761078.
Article28. Srinivasan B, Kolli AR, Esch MB, et al. TEER measurement techniques for in vitro barrier model systems. J Lab Autom. 2015; 20:107–126. PMID: 25586998.
Article29. Wilhelm I, Fazakas C, Krizbai IA. In vitro models of the blood-brain barrier. Acta Neurobiol Exp (Wars). 2011; 71:113–128. PMID: 21499332.30. Green LC, Wagner DA, Glogowski J, Tannenbaum SR, et al. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982; 126:131–138. PMID: 7181105.
Article31. Ammar DA, Hamweyah KM, Kahook MY. Antioxidants protect trabecular meshwork cells from hydrogen peroxide-induced cell death. Transl Vis Sci Technol. 2012; 1:4.
Article32. Wang H, Joseph JA. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic Biol Med. 1999; 27:612–616. PMID: 10490282.33. Alvarado JA, Wood I, Polansky JR. Human trabecular cells. II. Growth pattern and ultrastructural characteristics. Invest Ophthalmol Vis Sci. 1982; 23:464–478. PMID: 7118506.34. Salama NN, Eddington ND, Fasano A. Tight junction modulation and its relationship to drug delivery. Adv Drug Deliv Rev. 2006; 58:15–28. PMID: 16517003.
Article35. Grierson I, Lee WR. Pressure-induced changes in the ultrastructure of the endothelium lining Schlemm's canal. Am J Ophthalmol. 1975; 80:863–884. PMID: 811121.
Article36. Epstein DL, Rohen JW. Morphology of the trabecular meshwork and inner-wall endothelium after cationized ferritin perfusion in the monkey eye. Invest Ophthalmol Vis Sci. 1991; 32:160–171. PMID: 1987099.37. Raviola G, Raviola E. Paracellular route of aqueous outflow in the trabecular meshwork and canal of Schlemm. A freeze-fracture study of the endothelial junctions in the sclerocorneal angel of the macaque monkey eye. Invest Ophthalmol Vis Sci. 1981; 21:52–72. PMID: 7251302.38. Ethier CR. The inner wall of Schlemm's canal. Exp Eye Res. 2002; 74:161–172. PMID: 11950226.39. Tripathi RC. Mechanism of the aqueous outflow across the trabecular wall of Schlemm's canal. Exp Eye Res. 1971; 11:116–121. PMID: 4108660.
Article40. Tarkkanen A, Niemi M. Enzyme histochemistry of the angle of the anterior chamber of the human eye. Acta Ophthalmol (Copenh). 1967; 45:93–99. PMID: 4292700.
Article41. Michel JB, Feron O, Sacks D, Michel T. Reciprocal regulation of endothelial nitric-oxide synthase by Ca2+-calmodulin and caveolin. J Biol Chem. 1997; 272:15583–15586. PMID: 9188442.
Article42. Ju H, Zou R, Venema VJ, Venema RC. Direct interaction of endothelial nitric-oxide synthase and caveolin-1 inhibits synthase activity. J Biol Chem. 1997; 272:18522–18525. PMID: 9228013.
Article43. Schubert W, Frank PG, Woodman SE, et al. Microvascular hyperpermeability in caveolin-1 (-/-) knock-out mice. Treatment with a specific nitric-oxide synthase inhibitor, L-NAME, restores normal microvascular permeability in Cav-1 null mice. J Biol Chem. 2002; 277:40091–40098. PMID: 12167625.44. Lei Y, Song M, Wu J, et al. eNOS activity in CAV1 knockout mouse eyes. Invest Ophthalmol Vis Sci. 2016; 57:2805–2813. PMID: 27228562.
Article45. Yu J, Bergaya S, Murata T, et al. Direct evidence for the role of caveolin-1 and caveolae in mechanotransduction and remodeling of blood vessels. J Clin Invest. 2006; 116:1284–1291. PMID: 16670769.
Article46. Parton RG, Simons K. The multiple faces of caveolae. Nat Rev Mol Cell Biol. 2007; 8:185–194. PMID: 17318224.
Article47. Garcia-Cardena G, Martasek P, Masters BS, et al. Dissecting the interaction between nitric oxide synthase (NOS) and caveolin. Functional significance of the nos caveolin binding domain in vivo. J Biol Chem. 1997; 272:25437–25440. PMID: 9325253.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Growth Effect of Minoxidil and Minoxidil Sulfate on Cultured Human Keratinocytes and Outer Root Sheath Cells
- Hypertrichosis in a Woman During Treatment with 3% Topical Minoxidil
- The Study for the Outflow Of Aqueous Humor after Injection of Dye into the Anterior Chamber
- The Effects of Minoxidil Versus Mitomycin C on the Filteing Surgery for Glaucoma in Rabbits
- The Effect of Interleukin-1alpha on Trabecular Outflow Resistance in Rat Eyes