Blood Res.
2020 Sep;55(3):139-145. 10.5045/br.2020.2020130.
Use of generic imatinib as first-line treatment in patients with chronic myeloid leukemia (CML): the GIMS (Glivec to Imatinib Switch) study
- Affiliations
-
- 1Oncology Unit, San Gerardo Hospital, ASST-Monza, Italy
- 2Hematology Division and Bone Marrow Unit, Ospedale San Gerardo, ASSTMonza, Monza, Italy
- 3Division of Hematology, Fondazione IRCCS Policlinico San Matteo, Pavia, Italy
- 4Hematology Division, Foundation IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan, Italy
- 5Hematology and Bone Marrow Transplant Unit, ASST Papa Giovanni XXIII, Bergamo, Italy
- 6Hematology Unit, Ospedale di Circolo, Varese, Italy
- 7Division of Hematology, ASST Grande Ospedale Metropolitano Niguarda, Milano, Italy
- 8Hematology and Transfusional Medicine Unit, ASST Fatebenefratelli Sacco, Milan, Italy
- 9Department of Hematology, ASST Spedali Civili di Brescia, Brescia, Italy
- 10Hematology and Bone Marrow Transplantation Unit, San Raffaele Scientific Institute, IRCCS Milano, Italy
- 11Oncology Department, ASST Lecco, Lecco, Italy
- 12Chair of Hematology, Unit of Blood Diseases and Stem Cell Transplantation, University of Brescia, ASST Spedali Civili di Brescia, Brescia, Italy
- 13Department of Hematology, Cancer Center, IRCCS Humanitas Research Hospital/Humanitas University, Rozzano, Italy
- 14Department of Medicine and Surgery, University of Milano-Bicocca, Italy
- 15Center of Biostatistics for Clinical Epidemiology, Department of Medicine and Surgery, University of Milano-Bicocca, Monza, Italy
- KMID: 2507053
- DOI: http://doi.org/10.5045/br.2020.2020130
Abstract
- Background
Generic formulations of imatinib mesylate have been introduced in Western Europe since 2017 to treat patients with chronic myeloid leukemia (CML). However, results on the safety and efficacy of generic formulations are contrasting. The aim of this study was to investigate the safety and efficacy of generic imatinib in CML patients treated in 12 Italian institutes.
Methods
This is an observational, retro-prospective analysis of patients with CML for whom the treatment was switched from brand to generic imatinib. We analyzed and compared the variation in quantitative PCR values before and after the switch, and the proportion of patients who maintained molecular response after changing from brand to generic imatinib. Adverse events (AEs) were also evaluated.
Results
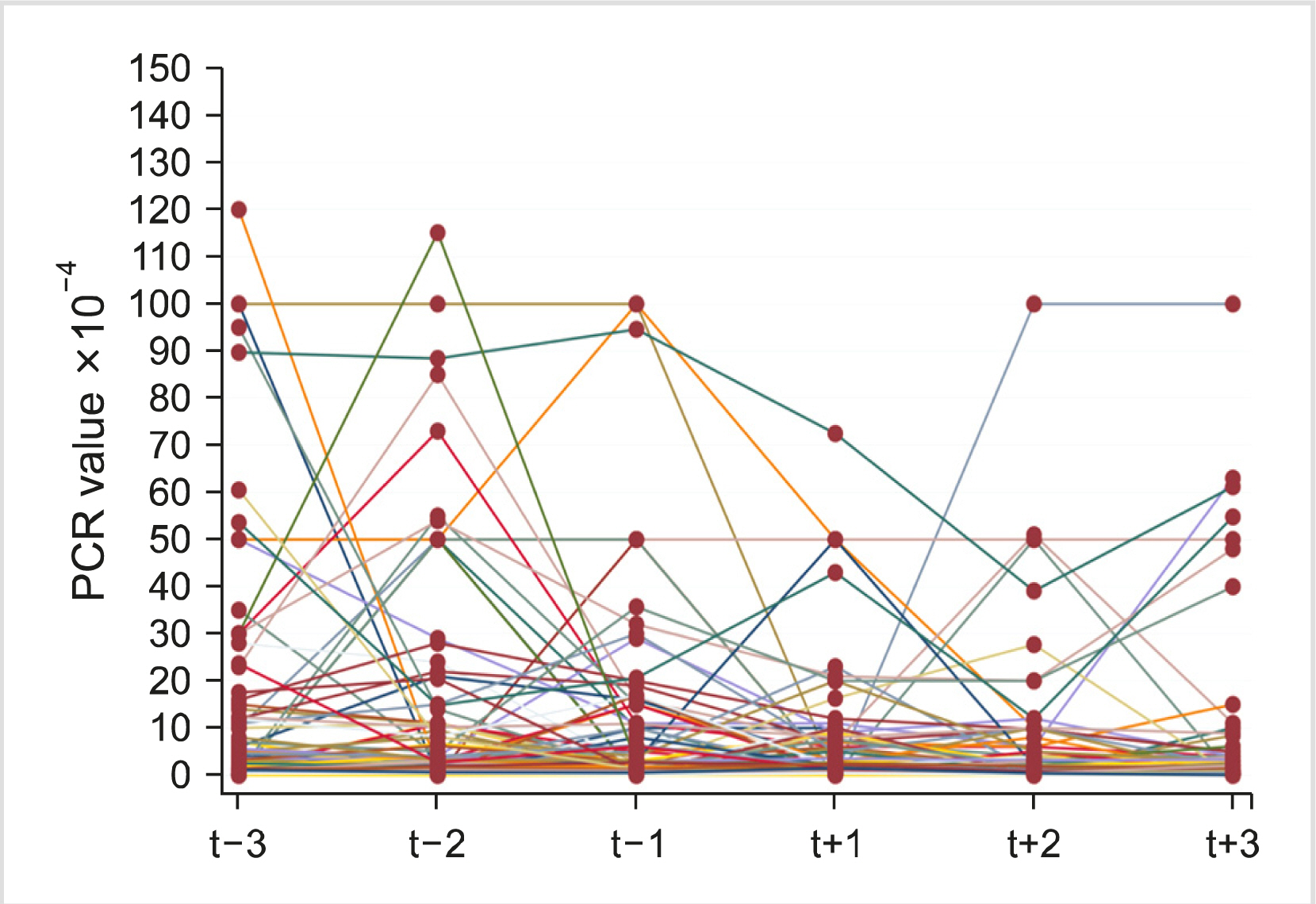
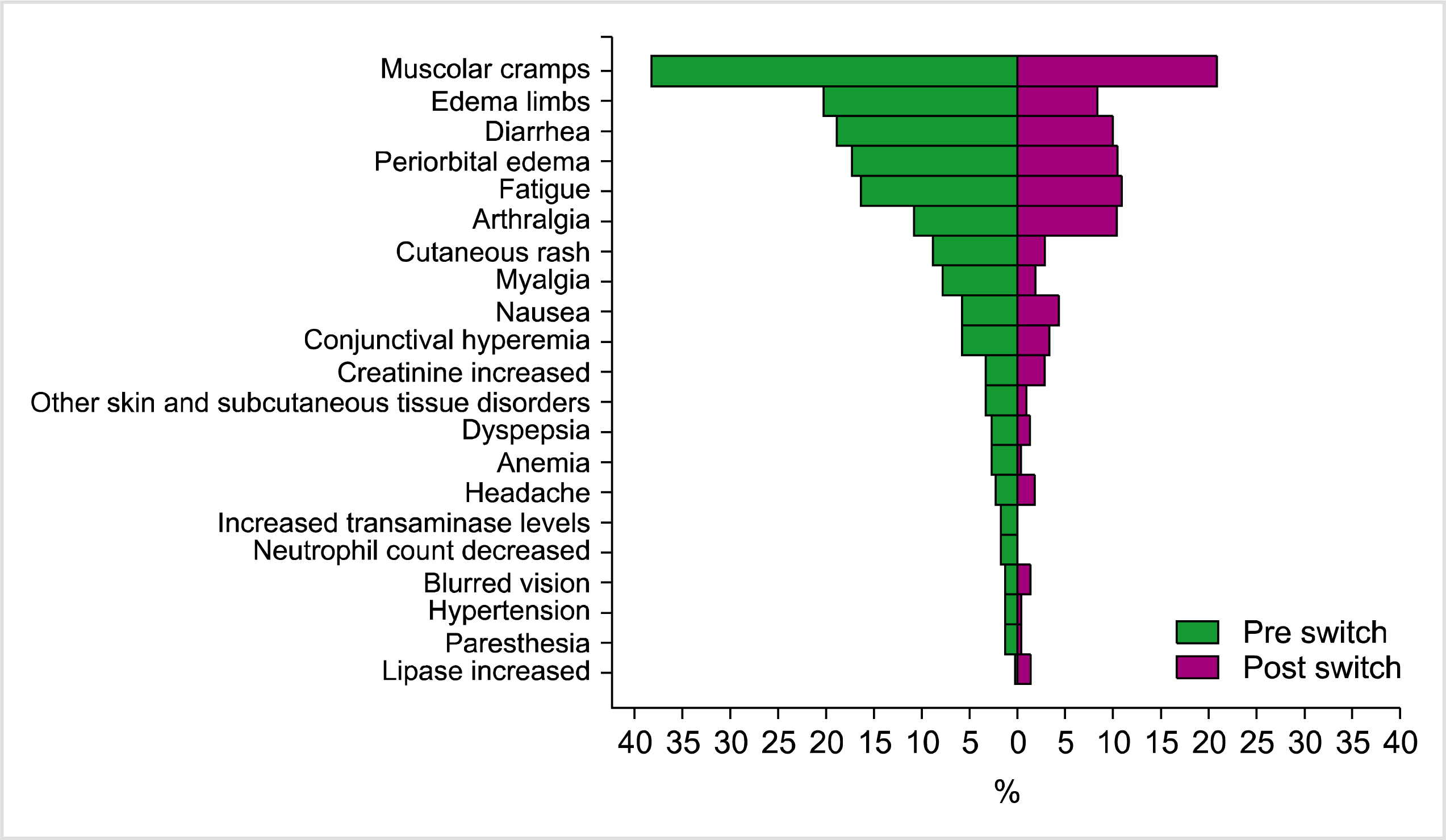
Two hundred patients were enrolled. The median PCR value after the switch was reduced by 0.25 compared to the values before the switch. A significant difference was found between median PCR values before and after the switch in favor of generic imatinib (P = 0 .0 0 3 ). Molecular responses remained stable in 69.0%, improved in 25.5%, and worsened in 5.5% of patients. AEs were similar in the pre- and post-switch periods; however, a significant difference was found in favor of generic imatinib for muscular cramps (P < 0.0001), periorbital edema (P =0.0028), edema of the limbs (P <0.0001), fatigue (P=0.0482), and diarrhea (P =0.0027).
Conclusion
Our data indicate that generic imatinib does not have deleterious effects on CML control and present an acceptable safety profile, similar or better than brand imatinib.
Figure
Reference
-
1. Rumpold H, Webersinke G. 2011; Molecular pathogenesis of Philadelphia- positive chronic myeloid leukemia - is it all BCR-ABL? Curr Cancer Drug Targets. 11:3–19. DOI: 10.2174/156800911793743619. PMID: 21062244.2. Kantarjian H, Sawyers C, Hochhaus A, et al. 2002; Hematologic and cytogenetic responses to imatinib mesylate in chronic myelogenous leukemia. N Engl J Med. 346:645–52. DOI: 10.1056/NEJMoa011573. PMID: 11870241.
Article3. O'Brien SG, Guilhot F, Larson RA, et al. 2003; Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 348:994–1004. DOI: 10.1056/NEJMoa022457. PMID: 12637609.4. Pfirrmann M, Baccarani M, Saussele S, et al. 2016; Prognosis of long-term survival considering disease-specific death in patients with chronic myeloid leukemia. Leukemia. 30:48–56. DOI: 10.1038/leu.2015.261. PMID: 26416462.
Article5. Mori S, Vagge E, le Coutre P, et al. 2015; Age and dPCR can predict relapse in CML patients who discontinued imatinib: the ISAV study. Am J Hematol. 90:910–4. DOI: 10.1002/ajh.24120. PMID: 26178642.
Article6. Yang YT, Nagai S, Chen BK, et al. 2016; Generic oncology drugs: are they all safe? Lancet Oncol. 17:e493–501. DOI: 10.1016/S1470-2045(16)30384-9. PMID: 27819247.
Article7. Vargas M, Villarraga E. 2017; Bioequivalence study of imatinib formulations that contain 400 mg in healthy colombians. J Bioequivalence Bioavailab. 9:483–8. DOI: 10.4172/jbb.1000349.8. Arora R, Sharma M, Monif T, Iyer S. 2016; A multi-centric bioequivalence trial in Ph+ chronic myeloid leukemia patients to assess bioequivalence and safety evaluation of generic imatinib mesylate 400 mg tablets. Cancer Res Treat. 48:1120–9. DOI: 10.4143/crt.2015.436. PMID: 26875198. PMCID: PMC4946363.
Article9. Ostojic A, Sertic D, Roncevic P, et al. 2016; Comparison of branded and generic imatinib plasma concentrations in patients with chronic myelogenous leukemia: unicentric study. Clin Lymphoma Myeloma Leuk. 16:472–6. DOI: 10.1016/j.clml.2016.04.003. PMID: 27245313.
Article10. Goubran HA. 2009; Failure of a non-authorized copy product to maintain response achieved with imatinib in a patient with chronic phase chronic myeloid leukemia: a case report. J Med Case Rep. 3:7112. DOI: 10.1186/1752-1947-3-7112. PMID: 19830137. PMCID: PMC2726486.
Article11. Mattar M. 2010; Failure of copy Imatib (CIPLA, India) to maintain hematologic and cytogenetic responses in chronic myeloid leukemia in chronic phase. Int J Hematol. 91:104–6. DOI: 10.1007/s12185-009-0431-1. PMID: 20054670.
Article12. Chouffai Z. 2010; Hematologic relapse after 2 years on a non-authorized copy version of imatinib in a patient with chronic myeloid leukemia in chronic phase: a case report. Case Rep Oncol. 3:272–6. DOI: 10.1159/000319150. PMID: 21045935. PMCID: PMC2968768.
Article13. Saavedra D, Vizcarra F. 2014; Deleterious effects of non-branded versions of imatinib used for the treatment of patients with chronic myeloid leukemia in chronic phase: a case series on an escalating issue impacting patient safety. Leuk Lymphoma. 55:2813–6. DOI: 10.3109/10428194.2014.893302. PMID: 24724785.
Article14. Alwan AF, Matti BF, Naji AS, Muhammed AH, Abdulsahib MA. 2014; Prospective single-center study of chronic myeloid leukemia in chronic phase: switching from branded imatinib to a copy drug and back. Leuk Lymphoma. 55:2830–4. DOI: 10.3109/10428194.2014.904508. PMID: 24628295.
Article15. Lejniece S, Udre I, Rivkina A. 2017; Generic imatinib in the treatment of chronic myeloid leukemia: two years' experience in Latvia. Exp Oncol. 39:151–4. DOI: 10.31768/2312-8852.2017.39(2):151-154. PMID: 29483494.
Article16. Awidi A, Abbasi S, Alrabi K, Kheirallah KA. 2017; Generic imatinib therapy among jordanians: an observational assessment of efficacy and safety in routine clinical practice. Clin Lymphoma Myeloma Leuk. 17:e55–61. DOI: 10.1016/j.clml.2017.08.001. PMID: 28844599.
Article17. Kesselheim AS, Gagne JJ, Eddings W, et al. 2016; Prevalence and predictors of generic drug skepticism among physicians: results of a national survey. JAMA Intern Med. 176:845–7. DOI: 10.1001/jamainternmed.2016.1688. PMID: 27158897.18. Kesselheim AS, Gagne JJ, Franklin JM, et al. 2016; Variations in patients' perceptions and use of generic drugs: results of a national survey. J Gen Intern Med. 31:609–14. DOI: 10.1007/s11606-016-3612-7. PMID: 27067349. PMCID: PMC4870431.
Article19. Geissler J, Sharf G, Cugurovic J, et al. 2016; Chronic myeloid leukemia patients call for quality and consistency when generics are introduced to treat their cancer. Leukemia. 30:2396–7. DOI: 10.1038/leu.2016.220. PMID: 27484147. PMCID: PMC5155028.
Article20. Klil-Drori AJ, Azoulay L, Yin H, et al. 2015; Comparative effectiveness of generic imatinib and brand-name imatinib for the treatment of chronic myeloid leukemia. Blood (ASH Annual Meeting Abstracts). 126(Suppl):2778. DOI: 10.1182/blood.V126.23.2778.2778.
Article21. Sacha T, Góra-Tybor J, Szarejko M, et al. 2017; A multicenter prospective study on efficacy and safety of imatinib generics: a report from Polish Adult Leukemia Group imatinib generics registry. Am J Hematol. 92:E125–8. DOI: 10.1002/ajh.24748. PMID: 28376561.
Article22. Ćojbašić I, Mačukanović-Golubović L, Vučić M, Ćojbašić Ž. 2019; Generic imatinib in chronic myeloid leukemia treatment: long-term follow-up. Clin Lymphoma Myeloma Leuk. 19:e526–31. DOI: 10.1016/j.clml.2019.05.006. PMID: 31239209.
Article23. Klil-Drori AJ, Yin H, Azoulay L, et al. 2019; Persistence with generic imatinib for chronic myeloid leukemia: a matched cohort study. Haematologica. 104:e293–5. DOI: 10.3324/haematol.2018.211235. PMID: 30630987. PMCID: PMC6601075.
Article24. Bonifacio M, Scaffidi L, Binotto G, et al. 2018; Safety and efficacy of switching from branded to generic imatinib in chronic phase chronic myeloid leukemia patients treated in Italy. Leuk Res. 74:75–9. DOI: 10.1016/j.leukres.2018.09.018. PMID: 30308414.
Article25. Eskazan AE, Ayer M, Kantarcioglu B, et al. 2014; First line treatment of chronic phase chronic myeloid leukaemia patients with the generic formulations of imatinib mesylate. Br J Haematol. 167:139–41. DOI: 10.1111/bjh.12937. PMID: 24815307.
Article26. Eskazan AE, Sadri S, Keskin D, et al. 2017; Outcomes of chronic myeloid leukemia patients with early molecular response at 3 and 6 months: a comparative analysis of generic imatinib and glivec. Clin Lymphoma Myeloma Leuk. 17:804–11. DOI: 10.1016/j.clml.2017.07.255. PMID: 28847475.
Article27. Entasoltan B, Bekadja MA, Touhami H, et al. 2017; Outcome of frontline treatment with "generic" imatinib in adult patients with chronic myeloid leukemia in algerian population: a multicenter study. Mediterr J Hematol Infect Dis. 9:e2017062. DOI: 10.4084/mjhid.2017.062. PMID: 29181139. PMCID: PMC5667527.
Article28. Islamagic E, Hasic A, Kurtovic S, et al. 2017; The efficacy of generic imatinib as first- and second-line therapy: 3-year follow-up of patients with chronic myeloid leukemia. Clin Lymphoma Myeloma Leuk. 17:238–40. DOI: 10.1016/j.clml.2017.02.001. PMID: 28283298.
Article29. Padula WV, Larson RA, Dusetzina SB, et al. 2016; Cost-effectiveness of tyrosine kinase inhibitor treatment strategies for chronic myeloid leukemia in chronic phase after generic entry of imatinib in the United States. J Natl Cancer Inst. 108:djw003. DOI: 10.1093/jnci/djw003. PMID: 26944912. PMCID: PMC4948567.
Article30. 2018; Generic drugs: are they the future for affordable medicine? Lancet Oncol. 19:149. DOI: 10.1016/S1470-2045(18)30033-0. PMID: 29413460.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Pilot with Chronic Myeloid Leukemia: Aeromedical Assessment
- Imatinib-Mesylate Induced Interstitial Pneumonitis in Two CML Patients
- Treatment-free remission after discontinuation of imatinib, dasatinib, and nilotinib in patients with chronic myeloid leukemia
- OCT-1, ABCB1, and ABCG2 Expression in Imatinib-Resistant Chronic Myeloid Leukemia Treated with Dasatinib or Nilotinib
- Three cases of Philadelphia (Ph) chromosome positive acute myeloid leukemia (AML) treated with imatinib mesylate (Glivec, STI571) and allogeneic hematopoietic stem cell transplantation after induction chemotherapy