Nutr Res Pract.
2020 Oct;14(5):438-452. 10.4162/nrp.2020.14.5.438.
Curcumin and hesperetin attenuate D-galactose-induced brain senescence in vitro and in vivo
- Affiliations
-
- 1Department of Nutritional Science and Food Management, Ewha Womans University, Seoul 03760, Korea.
- KMID: 2506882
- DOI: http://doi.org/10.4162/nrp.2020.14.5.438
Abstract
- BACKGROUND/OBJECTIVES
Brain senescence causes cognitive impairment and neurodegeneration. It has also been demonstrated that curcumin (Cur) and hesperetin (Hes), both antioxidant polyphenolic compounds, mediate anti-aging and neuroprotective effects. Therefore, the objective of this study was to investigate whether Cur, Hes, and/or their combination exert anti-aging effects in D-galactose (Dg)-induced aged neuronal cells and rats.
MATERIALS/METHODS
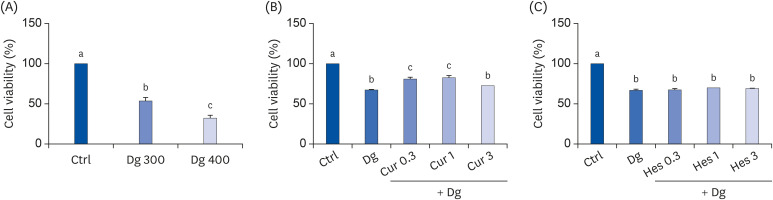
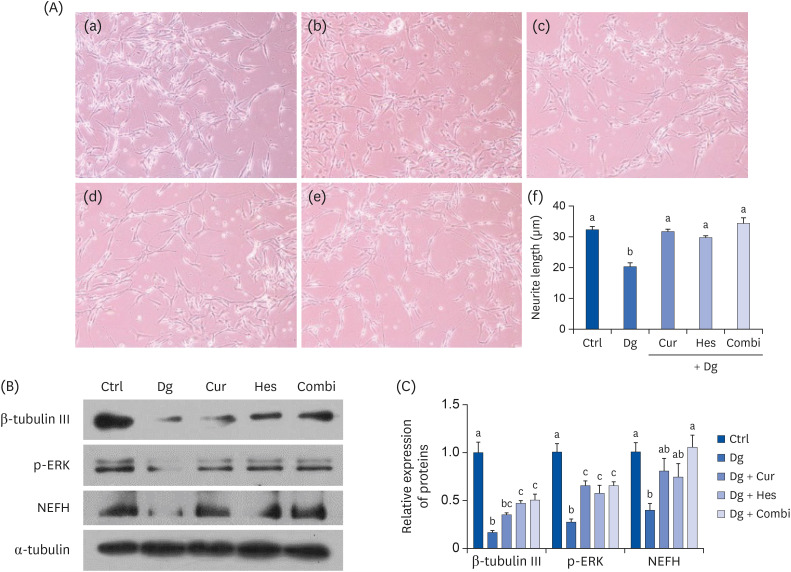
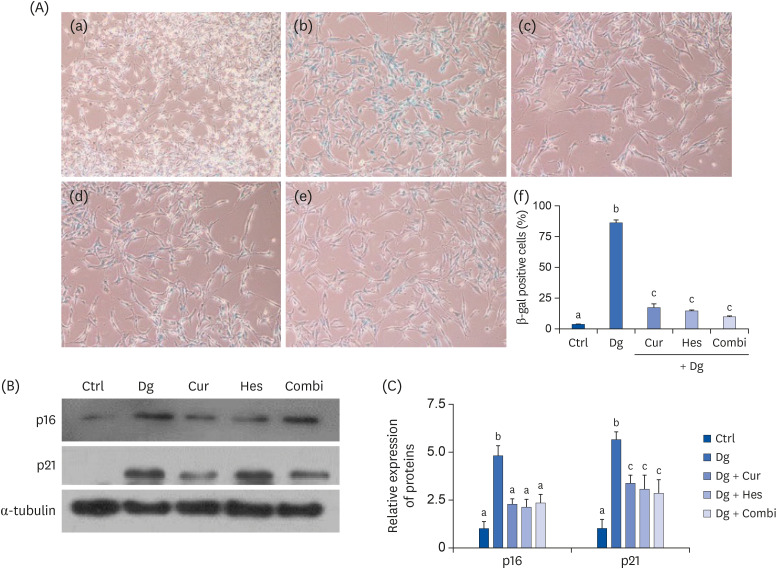
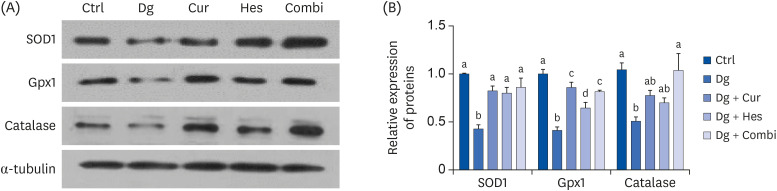
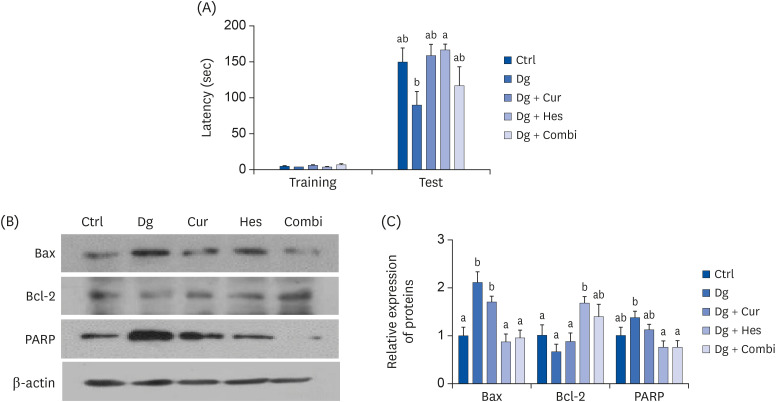
SH-SY5Y cells differentiated in response to retinoic acid were treated with Cur (1 μM), Hes (1 μM), or a combination of both, followed by 300 mM Dg. Neuronal loss was subsequently evaluated by measuring average neurite length and analyzing expression of β-tubulin III, phosphorylated extracellular signal-regulated kinases, and neurofilament heavy polypeptide. Cellular senescence and related proteins, p16 and p21, were also investigated, including their regulation of antioxidant enzymes. In vivo, brain aging was induced by injecting 250 mg/kg body weight (b.w.) Dg. The effects of supplementing this model with 50 mg/kg b.w. Cur, 50 mg/kg b.w. Hes, or a combination of both for 3 months were subsequently evaluated. Brain aging was examined with a step-through passive avoidance test and apoptosis markers were analyzed in brain cortex tissues.
RESULTS
Cur, Hes, and their combination improved neuron length and cellular senescence by decreasing the number of β-gal stained cells, down-regulated expression of p16 and p21, and up-regulated expression of antioxidant enzymes, including superoxide dismutase 1, glutathione peroxidase 1, and catalase. Administration of Cur, Hes, or their combination also tended to ameliorate cognitive impairment and suppress apoptosis in the cerebral cortex by downregulating Bax and poly (ADP-ribose) polymerase expression and increasing Bcl-2 expression.
CONCLUSIONS
Cur and Hes appear to attenuate Dg-induced brain aging via regulation of antioxidant enzymes and apoptosis. These results suggest that Cur and Hes may mediate neuroprotective effects in the aging process, and further study of these antioxidant polyphenolic compounds is warranted.
Keyword
Figure
Cited by 1 articles
-
Regulation of Cellular Senescence in Type 2 Diabetes Mellitus: From Mechanisms to Clinical Applications
Kanako Iwasaki, Cristian Abarca, Cristina Aguayo-Mazzucato
Diabetes Metab J. 2023;47(4):441-453. doi: 10.4093/dmj.2022.0416.
Reference
-
1. López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013; 153:1194–1217. PMID: 23746838.
Article3. Shwe T, Pratchayasakul W, Chattipakorn N, Chattipakorn SC. Role of D-galactose-induced brain aging and its potential used for therapeutic interventions. Exp Gerontol. 2018; 101:13–36. PMID: 29129736.
Article4. Stites SD, Harkins K, Rubright JD, Karlawish J. Relationships between cognitive complaints and quality of life in older adults with mild cognitive impairment, mild Alzheimer disease dementia, and normal cognition. Alzheimer Dis Assoc Disord. 2018; 32:276–283. PMID: 29944474.
Article5. Fjell AM, McEvoy L, Holland D, Dale AM, Walhovd KB. Alzheimer's Disease Neuroimaging Initiative. What is normal in normal aging? Effects of aging, amyloid and Alzheimer's disease on the cerebral cortex and the hippocampus. Prog Neurobiol. 2014; 117:20–40. PMID: 24548606.
Article6. Rubin RD, Watson PD, Duff MC, Cohen NJ. The role of the hippocampus in flexible cognition and social behavior. Front Hum Neurosci. 2014; 8:742. PMID: 25324753.
Article7. Crews L, Masliah E. Molecular mechanisms of neurodegeneration in Alzheimer's disease. Hum Mol Genet. 2010; 19:R12–20. PMID: 20413653.
Article8. Espuny-Camacho I, Arranz AM, Fiers M, Snellinx A, Ando K, Munck S, Bonnefont J, Lambot L, Corthout N, Omodho L, Eynden EV, Radaelli E, Tesseur I, Wray S, Ebneth A, Hardy J, Leroy K, Brion JP, Vanderhaeghen P, De Strooper B. Hallmarks of Alzheimer's disease in stem-cell-derived human neurons transplanted into mouse brain. Neuron. 2017; 93:1066–1081.e8. PMID: 28238547.
Article10. Chen P, Chen F, Zhou B. Antioxidative, anti-inflammatory and anti-apoptotic effects of ellagic acid in liver and brain of rats treated by D-galactose. Sci Rep. 2018; 8:1465. PMID: 29362375.
Article11. Shan Q, Lu J, Zheng Y, Li J, Zhou Z, Hu B, Zhang Z, Fan S, Mao Z, Wang YJ, Ma D. Purple sweet potato color ameliorates cognition deficits and attenuates oxidative damage and inflammation in aging mouse brain induced by D-galactose. J Biomed Biotechnol. 2009; 2009:564737. PMID: 19865488.
Article12. Kim Y, Kim E, Kim Y. L-histidine and L-carnosine accelerate wound healing via regulation of corticosterone and PI3K/Akt phosphorylation in D-galactose-induced aging models in vitro and in vivo . J Funct Foods. 2019; 58:227–237.13. Chen X, Li Y, Chen W, Nong Z, Huang J, Chen C. Protective effect of hyperbaric oxygen on cognitive impairment induced by D-galactose in mice. Neurochem Res. 2016; 41:3032–3041. PMID: 27485714.
Article14. Spencer JPE. Flavonoids: modulators of brain function? Br J Nutr. 2008; 99:ES60–ES77. PMID: 18503736.
Article15. Rehman SU, Shah SA, Ali T, Chung JI, Kim MO. Anthocyanins reversed D-galactose-induced oxidative stress and neuroinflammation mediated cognitive impairment in adult rats. Mol Neurobiol. 2017; 54:255–271. PMID: 26738855.
Article16. Hsieh HM, Wu WM, Hu ML. Soy isoflavones attenuate oxidative stress and improve parameters related to aging and Alzheimer's disease in C57BL/6J mice treated with D-galactose. Food Chem Toxicol. 2009; 47:625–632. PMID: 19146912.
Article17. Ritchie K, Carrière I, de Mendonça A, Portet F, Dartigues JF, Rouaud O, Barberger-Gateau P, Ancelin ML. The neuroprotective effects of caffeine: a prospective population study (the three city study). Neurology. 2007; 69:536–545. PMID: 17679672.
Article18. Tsuda T. Curcumin as a functional food-derived factor: degradation products, metabolites, bioactivity, and future perspectives. Food Funct. 2018; 9:705–714. PMID: 29206254.
Article19. Shen LR, Parnell LD, Ordovas JM, Lai CQ. Curcumin and aging. Biofactors. 2013; 39:133–140. PMID: 23325575.
Article20. Liao VH, Yu CW, Chu YJ, Li WH, Hsieh YC, Wang TT. Curcumin-mediated lifespan extension in Caenorhabditis elegans . Mech Ageing Dev. 2011; 132:480–487. PMID: 21855561.21. Lee KS, Lee BS, Semnani S, Avanesian A, Um CY, Jeon HJ, Seong KM, Yu K, Min KJ, Jafari M. Curcumin extends life span, improves health span, and modulates the expression of age-associated aging genes in Drosophila melanogaster. Rejuvenation Res. 2010; 13:561–570. PMID: 20645870.
Article22. Kitani K, Osawa T, Yokozawa T. The effects of tetrahydrocurcumin and green tea polyphenol on the survival of male C57BL/6 mice. Biogerontology. 2007; 8:567–573. PMID: 17516143.
Article23. Banji D, Banji OJF, Dasaroju S, Kranthi KCH. Curcumin and piperine abrogate lipid and protein oxidation induced by D-galactose in rat brain. Brain Res. 2013; 1515:1–11. PMID: 23566814.
Article24. Liu Y, Liu D, Zhu L, Gan Q, Le X. Temperature-dependent structure stability and in vitro release of chitosan-coated curcumin liposome. Food Res Int. 2015; 74:97–105. PMID: 28412008.25. Abdul Manap AS, Wei Tan AC, Leong WH, Yin Chia AY, Vijayabalan S, Arya A, Wong EH, Rizwan F, Bindal U, Koshy S, Madhavan P. Synergistic effects of curcumin and piperine as potent acetylcholine and amyloidogenic inhibitors with significant neuroprotective activity in SH-SY5Y cells via computational molecular modeling and in vitro assay. Front Aging Neurosci. 2019; 11:206. PMID: 31507403.
Article26. Cho J. Antioxidant and neuroprotective effects of hesperidin and its aglycone hesperetin. Arch Pharm Res. 2006; 29:699–706. PMID: 16964766.
Article27. Spencer JP, Vauzour D, Rendeiro C. Flavonoids and cognition: the molecular mechanisms underlying their behavioural effects. Arch Biochem Biophys. 2009; 492:1–9. PMID: 19822127.
Article28. Hirata A, Murakami Y, Shoji M, Kadoma Y, Fujisawa S. Kinetics of radical-scavenging activity of hesperetin and hesperidin and their inhibitory activity on COX-2 expression. Anticancer Res. 2005; 25:3367–3374. PMID: 16101151.29. Khalili M, Roghani M, Ekhlasi M. The effect of aqueous crocus sativus L. extract on intracerebroventricular streptozotocin-induced cognitive deficits in rat: a behavioral analysis. Iran J Pharm Res. 2009; 8:185–191.30. Quillfeldt JA. Behavioral methods to study learning and memory in rats. In : Andersen M, Tufik S, editors. Rodent Model as Tools in Ethical Biomedical Research. Cham: Springer;2016. p. 271–311.31. Rahnama S, Rabiei Z, Alibabaei Z, Mokhtari S, Rafieian-Kopaei M, Deris F. Anti-amnesic activity of Citrus aurantium flowers extract against scopolamine-induced memory impairments in rats. Neurol Sci. 2015; 36:553–560. PMID: 25367404.32. Song X, Bao M, Li D, Li YM. Advanced glycation in D-galactose induced mouse aging model. Mech Ageing Dev. 1999; 108:239–251. PMID: 10405984.
Article33. Shen Y, Gao H, Shi X, Wang N, Ai D, Li J, Ouyang L, Yang J, Tian Y, Lu J. Glutamine synthetase plays a role in D-galactose-induced astrocyte aging in vitro and in vivo. Exp Gerontol. 2014; 58:166–173. PMID: 25128847.34. Lin WL, Wang SM, Ho YJ, Kuo HC, Lee YJ, Tseng TH. Ethyl acetate extract of Wedelia chinensis inhibits tert-butyl hydroperoxide-induced damage in PC12 cells and D-galactose-induced neuronal cell loss in mice. BMC Complement Altern Med. 2014; 14:491. PMID: 25510435.
Article35. Wang T, Di G, Yang L, Dun Y, Sun Z, Wan J, Peng B, Liu C, Xiong G, Zhang C, Yuan D. Saponins from Panax japonicus attenuate D-galactose-induced cognitive impairment through its anti-oxidative and anti-apoptotic effects in rats. J Pharm Pharmacol. 2015; 67:1284–1296. PMID: 25892055.36. Rahimi VB, Askari VR, Mousavi SH. Ellagic acid reveals promising anti-aging effects against D-galactose-induced aging on human neuroblastoma cell line, SH-SY5Y: a mechanistic study. Biomed Pharmacother. 2018; 108:1712–1724. PMID: 30372874.
Article37. Ullah F, Ali T, Ullah N, Kim MO. Caffeine prevents D-galactose-induced cognitive deficits, oxidative stress, neuroinflammation and neurodegeneration in the adult rat brain. Neurochem Int. 2015; 90:114–124. PMID: 26209154.
Article38. Gao J, He H, Jiang W, Chang X, Zhu L, Luo F, Zhou R, Ma C, Yan T. Salidroside ameliorates cognitive impairment in a D-galactose-induced rat model of Alzheimer's disease. Behav Brain Res. 2015; 293:27–33. PMID: 26192909.
Article39. Kodali M, Parihar VK, Hattiangady B, Mishra V, Shuai B, Shetty AK. Resveratrol prevents age-related memory and mood dysfunction with increased hippocampal neurogenesis and microvasculature, and reduced glial activation. Sci Rep. 2015; 5:8075. PMID: 25627672.
Article40. Alharbi MH, Lamport DJ, Dodd GF, Saunders C, Harkness L, Butler LT, Spencer JP. Flavonoid-rich orange juice is associated with acute improvements in cognitive function in healthy middle-aged males. Eur J Nutr. 2016; 55:2021–2029. PMID: 26280945.
Article41. Sikora E, Scapagnini G, Barbagallo M. Curcumin, inflammation, ageing and age-related diseases. Immun Ageing. 2010; 7:1. PMID: 20205886.
Article42. Jurenka JS. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: a review of preclinical and clinical research. Altern Med Rev. 2009; 14:141–153. PMID: 19594223.43. Ringman JM, Frautschy SA, Teng E, Begum AN, Bardens J, Beigi M, Gylys KH, Badmaev V, Heath DD, Apostolova LG, Porter V, Vanek Z, Marshall GA, Hellemann G, Sugar C, Masterman DL, Montine TJ, Cummings JL, Cole GM. Oral curcumin for Alzheimer's disease: tolerability and efficacy in a 24-week randomized, double blind, placebo-controlled study. Alzheimers Res Ther. 2012; 4:43. PMID: 23107780.
Article44. Baum L, Lam CWK, Cheung SK, Kwok T, Lui V, Tsoh J, Lam L, Leung V, Hui E, Ng C, Woo J, Chiu HF, Goggins WB, Zee BC, Cheng KF, Fong CY, Wong A, Mok H, Chow MS, Ho PC, Ip SP, Ho CS, Yu XW, Lai CY, Chan MH, Szeto S, Chan IH, Mok V. Six-month randomized, placebo-controlled, double-blind, pilot clinical trial of curcumin in patients with Alzheimer disease. J Clin Psychopharmacol. 2008; 28:110–113. PMID: 18204357.
Article45. Cui Q, Li X, Zhu H. Curcumin ameliorates dopaminergic neuronal oxidative damage via activation of the Akt/Nrf2 pathway. Mol Med Rep. 2016; 13:1381–1388. PMID: 26648392.
Article46. Banji OJ, Banji D, Ch K. Curcumin and hesperidin improve cognition by suppressing mitochondrial dysfunction and apoptosis induced by D-galactose in rat brain. Food Chem Toxicol. 2014; 74:51–59. PMID: 25217884.
Article47. Banji OJ, Banji D, Soumya N, Chilipi KK, Kalpana CH, Kranthi Kumar CH, Annamalai AR. Combination of carvacrol with methotrexate suppresses Complete Freund's Adjuvant induced synovial inflammation with reduced hepatotoxicity in rats. Eur J Pharmacol. 2014; 723:91–98. PMID: 24333217.
Article48. Rainey-Smith S, Schroetke LW, Bahia P, Fahmi A, Skilton R, Spencer JP, Rice-Evans C, Rattray M, Williams RJ. Neuroprotective effects of hesperetin in mouse primary neurones are independent of CREB activation. Neurosci Lett. 2008; 438:29–33. PMID: 18467030.
Article49. Parhiz H, Roohbakhsh A, Soltani F, Rezaee R, Iranshahi M. Antioxidant and anti-inflammatory properties of the citrus flavonoids hesperidin and hesperetin: an updated review of their molecular mechanisms and experimental models. Phytother Res. 2015; 29:323–331. PMID: 25394264.
Article50. Roohbakhsh A, Parhiz H, Soltani F, Rezaee R, Iranshahi M. Neuropharmacological properties and pharmacokinetics of the citrus flavonoids hesperidin and hesperetin--a mini-review. Life Sci. 2014; 113:1–6. PMID: 25109791.51. Hwang SL, Yen GC. Neuroprotective effects of the citrus flavanones against H2O2-induced cytotoxicity in PC12 cells. J Agric Food Chem. 2008; 56:859–864. PMID: 18189359.
Article52. Huang SM, Tsai SY, Lin JA, Wu CH, Yen GC. Cytoprotective effects of hesperetin and hesperidin against amyloid β-induced impairment of glucose transport through downregulation of neuronal autophagy. Mol Nutr Food Res. 2012; 56:601–609. PMID: 22383310.
Article53. Nasser TIN, Spencer GE. Neurite outgrowth. Reference Module in Biomedical Sciences. Amsterdam: Elsevier;2017.54. Kashyap G, Bapat D, Das D, Gowaikar R, Amritkar RE, Rangarajan G, Ravindranath V, Ambika G. Synapse loss and progress of Alzheimer's disease -a network model. Sci Rep. 2019; 9:6555. PMID: 31024073.
Article55. Zhu J, Mu X, Zeng J, Xu C, Liu J, Zhang M, Li C, Chen J, Li T, Wang Y. Ginsenoside Rg1 prevents cognitive impairment and hippocampus senescence in a rat model of D-galactose-induced aging. PLoS One. 2014; 9:e101291. PMID: 24979747.
Article56. Elzi DJ, Song M, Shiio Y. Role of galactose in cellular senescence. Exp Gerontol. 2016; 73:1–4. PMID: 26547052.
Article57. Nopparat C, Chantadul V, Permpoonputtana K, Govitrapong P. The anti-inflammatory effect of melatonin in SH-SY5Y neuroblastoma cells exposed to sublethal dose of hydrogen peroxide. Mech Ageing Dev. 2017; 164:49–60. PMID: 28408139.
Article58. Justin Thenmozhi A, William Raja TR, Manivasagam T, Janakiraman U, Essa MM. Hesperidin ameliorates cognitive dysfunction, oxidative stress and apoptosis against aluminium chloride induced rat model of Alzheimer's disease. Nutr Neurosci. 2017; 20:360–368. PMID: 26878879.
Article59. Dimpfel W. Different anticonvulsive effects of hesperidin and its aglycone hesperetin on electrical activity in the rat hippocampus in-vitro. J Pharm Pharmacol. 2006; 58:375–379. PMID: 16536905.
Article60. Kumar A, Dogra S, Prakash A. Effect of carvedilol on behavioral, mitochondrial dysfunction, and oxidative damage against D-galactose induced senescence in mice. Naunyn Schmiedebergs Arch Pharmacol. 2009; 380:431–441. PMID: 19685040.
Article61. Metzler M, Pfeiffer E, Schulz SI, Dempe JS. Curcumin uptake and metabolism. Biofactors. 2013; 39:14–20. PMID: 22996406.
Article62. Kanaze FI, Bounartzi MI, Georgarakis M, Niopas I. Pharmacokinetics of the citrus flavanone aglycones hesperetin and naringenin after single oral administration in human subjects. Eur J Clin Nutr. 2007; 61:472–477. PMID: 17047689.
Article63. Ghosh SS, He H, Wang J, Gehr TW, Ghosh S. Curcumin-mediated regulation of intestinal barrier function: the mechanism underlying its beneficial effects. Tissue Barriers. 2018; 6:e1425085. PMID: 29420166.
Article64. Trivedi PP, Tripathi DN, Jena GB. Hesperetin protects testicular toxicity of doxorubicin in rat: role of NFκB, p38 and caspase-3. Food Chem Toxicol. 2011; 49:838–847. PMID: 21168534.
Article65. Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008; 22:659–661. PMID: 17942826.66. Mishra S, Palanivelu K. The effect of curcumin (turmeric) on Alzheimer's disease: an overview. Ann Indian Acad Neurol. 2008; 11:13–19. PMID: 19966973.67. Bielak-Zmijewska A, Grabowska W, Ciolko A, Bojko A, Mosieniak G, Bijoch Ł, Sikora E. The role of curcumin in the modulation of ageing. Int J Mol Sci. 2019; 20:1239.
Article68. Mouly P, Gaydou EM, Auffray A. Simultaneous separation of flavanone glycosides and polymethoxylated flavones in citrus juices using liquid chromatography. J Chromatogr A. 1998; 800:171–179. PMID: 9561761.
Article69. Erlund I, Silaste ML, Alfthan G, Rantala M, Kesäniemi YA, Aro A. Plasma concentrations of the flavonoids hesperetin, naringenin and quercetin in human subjects following their habitual diets, and diets high or low in fruit and vegetables. Eur J Clin Nutr. 2002; 56:891–898. PMID: 12209378.
Article70. Cheng AL, Hsu CH, Lin JK, Hsu MM, Ho YF, Shen TS, Ko JY, Lin JT, Lin BR, Ming-Shiang W, Yu HS, Jee SH, Chen GS, Chen TM, Chen CA, Lai MK, Pu YS, Pan MH, Wang YJ, Tsai CC, Hsieh CY. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001; 21:2895–2900. PMID: 11712783.71. Garcia-Alloza M, Borrelli LA, Rozkalne A, Hyman BT, Bacskai BJ. Curcumin labels amyloid pathology in vivo, disrupts existing plaques, and partially restores distorted neurites in an Alzheimer mouse model. J Neurochem. 2007; 102:1095–1104. PMID: 17472706.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Curcumin-Induced Autophagy Augments Its Antitumor Effect against A172 Human Glioblastoma Cells
- Inhibitory effects of curcumin on passive cutaneous anaphylactoid response and compound 48/80-induced mast cell activation
- Recent Developments in Delivery, Bioavailability, Absorption and Metabolism of Curcumin: the Golden Pigment from Golden Spice
- L-histidine and L-carnosine exert anti-brain aging effects in D-galactose-induced aged neuronal cells
- Antipruritic effect of curcumin on histamine-induced itching in mice