Korean J Gastroenterol.
2020 Sep;76(3):108-133. 10.4166/kjg.2020.76.3.108.
Clinical Guidelines for Drug-induced Peptic Ulcer, 2020 Revised Edition
- Affiliations
-
- 1Department of Internal Medicine, Korea University Guro Hospital, Korea University College of Medicine, Seoul, Korea
- 2Department of Internal Medicine, Hanyang University Guri Hospital, Hanyang University College of Medicine, Guri, Korea
- 3Department of Internal Medicine, Incheon St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 4Department of Internal Medicine, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 5Department of Gastroenterology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 6Department of Internal Medicine, Pusan National University School of Medicine, Busan, Korea
- 7Division of Gastroenterology, Department of Internal Medicine, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea
- 8Department of Internal Medicine, Hallym University College of Medicine, Chuncheon, Korea
- 9Department of Internal Medicine, Chung-Ang University College of Medicine, Seoul, Korea
- 10Department of Internal Medicine, College of Medicine, Ewha Woman’s University, Seoul, Korea
- 11Department of Internal Medicine, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea
- KMID: 2506668
- DOI: http://doi.org/10.4166/kjg.2020.76.3.108
Abstract
- The Korean guidelines for nonsteroidal anti-inflammatory drug (NSAID)-induced peptic ulcers were previously developed under co-work with the Korean College of Helicobacter and Upper Gastrointestinal Research and Korean Society of Gastroenterology at 2009. On the other hand, the previous guidelines were based mainly on a literature review and expert opinions. Therefore, the guidelines need to be revised. In this study, a guideline development committee for drug-induced peptic ulcers was organized under the Korean College of Helicobacter and Upper Gastrointestinal Research in 2017. Nine statements were developed, including four for NSAID, three for aspirin and other antiplatelet agents, and two for anticoagulants through de novo processes based on evidence-based medicine, such as a literature search, meta-analysis, and the consensus was established using the modified Delphi method. The primary target of this guideline was adult patients taking long-term NSAIDs, aspirin, or other antiplatelet agent and anticoagulants. The revised guidelines reflect the consensus of expert opinions and are intended to assist relevant clinicians in the management and prevention of drug-induced peptic ulcers and associated conditions.
Keyword
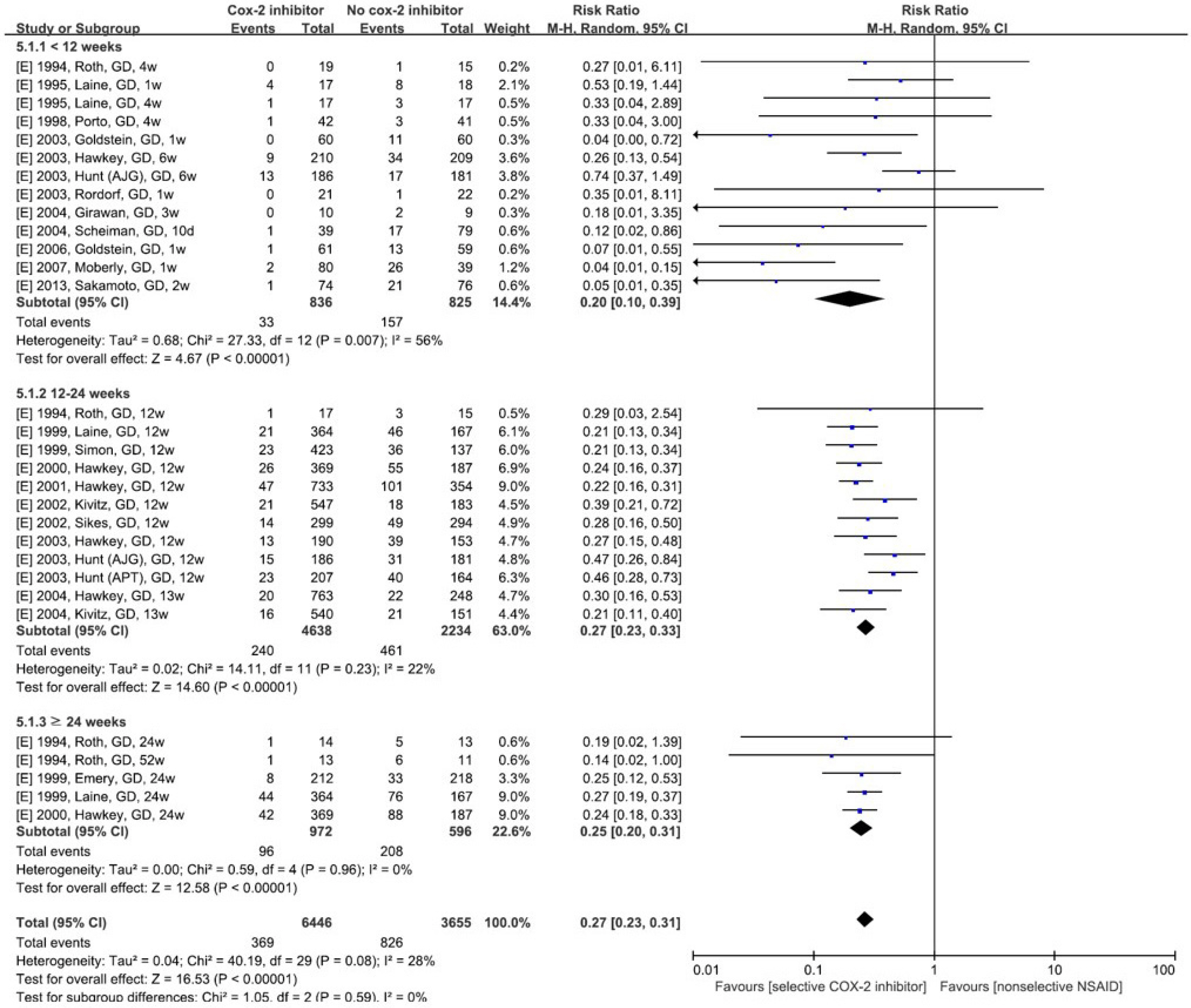
Figure
Cited by 3 articles
-
Helicobacter pylori Eradication in Drug-related Peptic Ulcer
Moon Kyung Joo
Korean J Gastroenterol. 2020;76(5):227-231. doi: 10.4166/kjg.2020.141.Prevention of Non-steroidal Anti-inflammatory Drug-induced Peptic Ulcers
Seong Jun Chu, Kyu-Tae Yoon, Joon Sung Kim
Korean J Gastroenterol. 2020;76(5):232-237. doi: 10.4166/kjg.2020.139.Prevention of Peptic Ulcer Associated with Aspirin and Antiplatelet Agent
Ji Yong Ahn
Korean J Gastroenterol. 2020;76(5):238-241. doi: 10.4166/kjg.2020.140.
Reference
-
1. Jhun HJ, Sung NJ, Kim SY. 2013; Knee pain and its severity in elderly Koreans: prevalence, risk factors and impact on quality of life. J Korean Med Sci. 28:1807–1813. DOI: 10.3346/jkms.2013.28.12.1807. PMID: 24339713. PMCID: PMC3857379.
Article2. Lee JH, Lim NK, Cho MC, Park HY. 2016; Epidemiology of heart failure in Korea: present and future. Korean Circ J. 46:658–664. DOI: 10.4070/kcj.2016.46.5.658. PMID: 27721857. PMCID: PMC5054178.
Article3. Shim YK, Kim N. 2016; Nonsteroidal anti-inflammatory drug and aspirin-induced peptic ulcer disease. Korean J Gastroenterol. 67:300–312. DOI: 10.4166/kjg.2016.67.6.300. PMID: 27312830. PMCID: PMC6857243.
Article4. Silverstein FE, Graham DY, Senior JR, et al. 1995; Misoprostol reduces serious gastrointestinal complications in patients with rheumatoid arthritis receiving nonsteroidal anti-inflammatory drugs. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 123:241–249. DOI: 10.7326/0003-4819-123-4-199508150-00001. PMID: 7611589.5. Silverstein FE, Faich G, Goldstein JL, et al. 2000; Gastrointestinal toxicity with celecoxib vs nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis: the CLASS study: a randomized controlled trial. Celecoxib long-term arthritis safety study. JAMA. 284:1247–1255. DOI: 10.1001/jama.284.10.1247. PMID: 10979111.6. Bombardier C, Laine L, Reicin A, et al. 2000; Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. VIGOR Study Group. N Engl J Med. 343:1520–1528. DOI: 10.1056/NEJM200011233432103. PMID: 11087881.7. Schnitzer TJ, Burmester GR, Mysler E, et al. 2004; Comparison of lumiracoxib with naproxen and ibuprofen in the Therapeutic Arthritis Research and Gastrointestinal Event Trial (TARGET), reduction in ulcer complications: randomised controlled trial. Lancet. 364:665–674. DOI: 10.1016/S0140-6736(04)16893-1. PMID: 15325831.
Article8. Bhatt DL, Fox KA, Hacke W, et al. 2006; Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med. 354:1706–1717. DOI: 10.1056/NEJMoa060989. PMID: 16531616. PMCID: PMC6486024.9. Moukarbel GV, Signorovitch JE, Pfeffer MA, et al. 2009; Gastrointestinal bleeding in high risk survivors of myocardial infarction: the VALIANT Trial. Eur Heart J. 30:2226–2232. DOI: 10.1093/eurheartj/ehp256. PMID: 19556260. PMCID: PMC5985637.
Article10. Kim JI, Kim SG, Kim N, et al. 2009; Changing prevalence of upper gastrointestinal disease in 28 893 Koreans from 1995 to 2005. Eur J Gastroenterol Hepatol. 21:787–793. DOI: 10.1097/MEG.0b013e32830e285a. PMID: 19404205.11. Yang YJ, Bang CS, Shin SP, et al. 2017; Clinical characteristics of peptic ulcer perforation in Korea. World J Gastroenterol. 23:2566–2574. DOI: 10.3748/wjg.v23.i14.2566. PMID: 28465641. PMCID: PMC5394520.
Article12. Lanza FL, Chan FK, Quigley EM. Practice Parameters Committee of the American College of Gastroenterology. 2009; Guidelines for prevention of NSAID-related ulcer complications. Am J Gastroenterol. 104:728–738. DOI: 10.1038/ajg.2009.115. PMID: 19240698.
Article13. Rostom A, Moayyedi P, Hunt R. Canadian Association of Gastroenterology Consensus Group. 2009; Canadian consensus guidelines on long-term nonsteroidal anti-inflammatory drug therapy and the need for gastroprotection: benefits versus risks. Aliment Pharmacol Ther. 29:481–496. DOI: 10.1111/j.1365-2036.2008.03905.x. PMID: 19053986.
Article14. Satoh K, Yoshino J, Akamatsu T, et al. 2016; Evidence-based clinical practice guidelines for peptic ulcer disease 2015. J Gastroenterol. 51:177–194. DOI: 10.1007/s00535-016-1166-4. PMID: 26879862. PMCID: PMC6308079.
Article15. Lee JH, Lee YC, Jeon SW, et al. 2009; Guidelines of prevention and treatment for NSAID-related peptic ulcers. Korean J Gastroenterol. 54:309–317. DOI: 10.4166/kjg.2009.54.5.309. PMID: 19934612.
Article16. Kim SG, Kim JG, Shin SK, et al. 2009; Guidelines of diagnosis for peptic ulcer disease. Korean J Gastroenterol. 54:279–284. DOI: 10.4166/kjg.2009.54.5.279. PMID: 19934609.
Article17. Cheung DY, Jung HY, Song HJ, et al. 2009; Guidelines of treatment for non-bleeding peptic ulcer disease. Korean J Gastroenterol. 54:285–297. DOI: 10.4166/kjg.2009.54.5.285. PMID: 19934610.
Article18. Chung IK, Lee DH, Kim HU, et al. 2009; Guidelines of treatment for bleeding peptic ulcer disease. Korean J Gastroenterol. 54:298–308. DOI: 10.4166/kjg.2009.54.5.298. PMID: 19934611.
Article19. Kim JH, Moon JS, Jee SR, et al. 2009; Guidelines of treatment for peptic ulcer disease in special conditions. Korean J Gastroenterol. 54:318–327. DOI: 10.4166/kjg.2009.54.5.318. PMID: 19934613. PMCID: PMC7386955.
Article20. Higgins JP, Altman DG, Sterne JA. 2011. Chapter 8: assessing the risk of bias in included studies. Cochrane Handbook for systematic reviews of interventions: The Cochrane collaboration. John Wiley & Sons;Chichester:21. Kim SY, Park JE, Lee YJ, et al. 2013; Testing a tool for assessing the risk of bias for nonrandomized studies showed moderate reliability and promising validity. J Clin Epidemiol. 66:408–414. DOI: 10.1016/j.jclinepi.2012.09.016. PMID: 23337781.
Article22. Kim SY, Choi MY, Shin SS, et al. 2015. NECA's handbook for clinical practice guideline developer version 1.0. National Evidence-based healthcare Collaborating Agency;Seoul:23. Shorr RI, Ray WA, Daugherty JR, Griffin MR. 1993; Concurrent use of nonsteroidal anti-inflammatory drugs and oral anticoagulants places elderly persons at high risk for hemorrhagic peptic ulcer disease. Arch Intern Med. 153:1665–1670. DOI: 10.1001/archinte.1993.00410140047006. PMID: 8333804.
Article24. García Rodríguez LA, Jick H. 1994; Risk of upper gastrointestinal bleeding and perforation associated with individual non-steroidal anti-inflammatory drugs. Lancet. 343:769–772. DOI: 10.1016/S0140-6736(94)91843-0. PMID: 7907735.
Article25. Lee HL, Han DS, Kim JB, et al. 2004; Importance of age and other risk factors in NSAID-induced gastropathy. Korean J Gastroenterol. 44:246–251. DOI: 10.1016/j.bpg.2009.10.004. PMID: 15564803.26. Lanas A, García-Rodríguez LA, Arroyo MT, et al. 2006; Risk of upper gastrointestinal ulcer bleeding associated with selective cyclo-oxygenase-2 inhibitors, traditional non-aspirin non-steroidal anti-inflammatory drugs, aspirin and combinations. Gut. 55:1731–1738. DOI: 10.1136/gut.2005.080754. PMID: 16687434. PMCID: PMC1856452.
Article27. Kobata Y, Yajima H, Yamao J, Tanaka Y, Fukui H, Takakura Y. 2009; Risk factors for the development of gastric mucosal lesions in rheumatoid arthritis patients receiving long-term nonsteroidal anti-inflammatory drug therapy and the efficacy of famotidine obtained from the FORCE study. Mod Rheumatol. 19:629–636. DOI: 10.3109/s10165-009-0202-0. PMID: 19728013.
Article28. Laine L, Curtis SP, Cryer B, Kaur A, Cannon CP. 2010; Risk factors for NSAID-associated upper GI clinical events in a long-term prospective study of 34 701 arthritis patients. Aliment Pharmacol Ther. 32:1240–1248. DOI: 10.1111/j.1365-2036.2010.04465.x. PMID: 20955443.29. Milder TY, Lipworth WL, Williams KM, Ritchie JE, Day RO. 2011; "It looks after me": how older patients make decisions about analgesics for osteoarthritis. Arthritis Care Res (Hoboken). 63:1280–1286. DOI: 10.1002/acr.20514. PMID: 21671423.
Article30. Chan FK, Sung JJ, Chung SC, et al. 1997; Randomised trial of eradication of Helicobacter pylori before non-steroidal anti-inflammatory drug therapy to prevent peptic ulcers. Lancet. 350:975–979. DOI: 10.1016/S0140-6736(97)04523-6. PMID: 9329511.
Article31. Chan FK, To KF, Wu JC, et al. 2002; Eradication of Helicobacter pylori and risk of peptic ulcers in patients starting long-term treatment with non-steroidal anti-inflammatory drugs: a randomised trial. Lancet. 359:9–13. DOI: 10.1016/S0140-6736(02)07272-0. PMID: 11809180.32. Labenz J, Blum AL, Bolten WW, et al. 2002; Primary prevention of diclofenac associated ulcers and dyspepsia by omeprazole or triple therapy in Helicobacter pylori positive patients: a randomised, double blind, placebo controlled, clinical trial. Gut. 51:329–335. DOI: 10.1136/gut.51.3.329. PMID: 12171952. PMCID: PMC1773346.
Article33. Hawkey CJ, Tulassay Z, Szczepanski L, et al. 1998; Randomised controlled trial of Helicobacter pylori eradication in patients on non-steroidal anti-inflammatory drugs: HELP NSAIDs study. Helicobacter Eradication for Lesion Prevention. Lancet. 352:1016–1021. DOI: 10.1016/S0140-6736(98)04206-8. PMID: 9759744. PMCID: PMC4588401.34. Lai KC, Lau CS, Ip WY, et al. 2003; Effect of treatment of Helicobacter pylori on the prevention of gastroduodenal ulcers in patients receiving long-term NSAIDs: a double-blind, placebo-controlled trial. Aliment Pharmacol Ther. 17:799–805. DOI: 10.1046/j.1365-2036.2003.01528.x. PMID: 12641502.
Article35. de Leest HT, Steen KS, Lems WF, et al. 2007; Eradication of Helicobacter pylori does not reduce the incidence of gastroduodenal ulcers in patients on long-term NSAID treatment: double-blind, randomized, placebo-controlled trial. Helicobacter. 12:477–485. DOI: 10.1111/j.1523-5378.2007.00543.x. PMID: 17760715.
Article36. Chan FK, Chung SC, Suen BY, et al. 2001; Preventing recurrent upper gastrointestinal bleeding in patients with Helicobacter pylori infection who are taking low-dose aspirin or naproxen. N Engl J Med. 344:967–973. DOI: 10.1056/NEJM200103293441304. PMID: 11274623.37. Malfertheiner P, Megraud F, O'Morain CA, et al. 2017; Management of Helicobacter pylori infection-the Maastricht V/Florence consensus report. Gut. 66:6–30. DOI: 10.1136/gutjnl-2016-312288. PMID: 27707777.38. Lee JW, Kim N, Kim JM, et al. 2013; Prevalence of primary and secondary antimicrobial resistance of Helicobacter pylori in Korea from 2003 through 2012. Helicobacter. 18:206–214. DOI: 10.1111/hel.12031. PMID: 23241101.39. Ekström P, Carling L, Wetterhus S, et al. 1996; Prevention of peptic ulcer and dyspeptic symptoms with omeprazole in patients receiving continuous non-steroidal anti-inflammatory drug therapy. A Nordic multicentre study. Scand J Gastroenterol. 31:753–758. DOI: 10.3109/00365529609010347. PMID: 8858742.
Article40. Bianchi Porro G, Lazzaroni M, Petrillo M, Manzionna G, Montrone F, Caruso I. 1998; Prevention of gastroduodenal damage with omeprazole in patients receiving continuous NSAIDs treatment. A double blind placebo controlled study. Ital J Gastroenterol Hepatol. 30:43–47. PMID: 9615264.41. Cullen D, Bardhan KD, Eisner M, et al. 1998; Primary gastroduodenal prophylaxis with omeprazole for non-steroidal anti-inflammatory drug users. Aliment Pharmacol Ther. 12:135–140. DOI: 10.1046/j.1365-2036.1998.00288.x. PMID: 9692687.
Article42. Bianchi Porro G, Lazzaroni M, Imbesi V, Montrone F, Santagada T. 2000; Efficacy of pantoprazole in the prevention of peptic ulcers, induced by non-steroidal anti-inflammatory drugs: a prospective, placebo-controlled, double-blind, parallel-group study. Dig Liver Dis. 32:201–208. DOI: 10.1016/S1590-8658(00)80821-X. PMID: 10975769.
Article43. Lai KC, Lam SK, Chu KM, et al. 2003; Lansoprazole reduces ulcer relapse after eradication of Helicobacter pylori in nonsteroidal anti-inflammatory drug users--a randomized trial. Aliment Pharmacol Ther. 18:829–836. DOI: 10.1046/j.1365-2036.2003.01762.x. PMID: 14535877.44. Scheiman JM, Yeomans ND, Talley NJ, et al. 2006; Prevention of ulcers by esomeprazole in at-risk patients using non-selective NSAIDs and COX-2 inhibitors. Am J Gastroenterol. 101:701–710. DOI: 10.1111/j.1572-0241.2006.00499.x. PMID: 16494585.
Article45. Desai JC, Sanyal SM, Goo T, et al. 2008; Primary prevention of adverse gastroduodenal effects from short-term use of non-steroidal anti-inflammatory drugs by omeprazole 20 mg in healthy subjects: a randomized, double-blind, placebo-controlled study. Dig Dis Sci. 53:2059–2065. DOI: 10.1007/s10620-007-0127-4. PMID: 18224442.46. Sugano K, Kinoshita Y, Miwa H, Takeuchi T. Esomeprazole NSAID Preventive Study Group. 2012; Randomised clinical trial: esomeprazole for the prevention of nonsteroidal anti-inflammatory drug-related peptic ulcers in Japanese patients. Aliment Pharmacol Ther. 36:115–125. DOI: 10.1111/j.1365-2036.2012.05133.x. PMID: 22591121.
Article47. Martin RM, Dunn NR, Freemantle S, Shakir S. 2000; The rates of common adverse events reported during treatment with proton pump inhibitors used in general practice in England: cohort studies. Br J Clin Pharmacol. 50:366–372. DOI: 10.1046/j.1365-2125.2000.00262.x. PMID: 11012560. PMCID: PMC2014999.
Article48. Gyawali CP, Fass R. 2018; Management of gastroesophageal reflux disease. Gastroenterology. 154:302–318. DOI: 10.1053/j.gastro.2017.07.049. PMID: 28827081. PMCID: PMC7502866.
Article49. Gray SL, LaCroix AZ, Larson J, et al. 2010; Proton pump inhibitor use, hip fracture, and change in bone mineral density in postmenopausal women: results from the Women's Health Initiative. Arch Intern Med. 170:765–771. DOI: 10.1001/archinternmed.2010.94. PMID: 20458083. PMCID: PMC4240017.50. Herzig SJ, Howell MD, Ngo LH, Marcantonio ER. 2009; Acid-suppressive medication use and the risk for hospital-acquired pneumonia. JAMA. 301:2120–2128. DOI: 10.1001/jama.2009.722. PMID: 19470989. PMCID: PMC7462971.
Article51. Howell MD, Novack V, Grgurich P, et al. 2010; Iatrogenic gastric acid suppression and the risk of nosocomial Clostridium difficile infection. Arch Intern Med. 170:784–790. DOI: 10.1001/archinternmed.2010.89. PMID: 20458086.
Article52. Park CH, Kim EH, Roh YH, Kim HY, Lee SK. 2014; The association between the use of proton pump inhibitors and the risk of hypomagnesemia: a systematic review and meta-analysis. PLoS One. 9:e112558. DOI: 10.1371/journal.pone.0112558. PMID: 25394217. PMCID: PMC4230950.
Article53. Sierra F, Suarez M, Rey M, Vela MF. 2007; Systematic review: proton pump inhibitor-associated acute interstitial nephritis. Aliment Pharmacol Ther. 26:545–553. DOI: 10.1111/j.1365-2036.2007.03407.x. PMID: 17661758.
Article54. Tai SY, Chien CY, Wu DC, et al. 2017; Risk of dementia from proton pump inhibitor use in Asian population: a nationwide cohort study in Taiwan. PLoS One. 12:e0171006. DOI: 10.1371/journal.pone.0171006. PMID: 28199356. PMCID: PMC5310771.
Article55. Shah NH, LePendu P, Bauer-Mehren A, et al. 2015; Proton pump inhibitor usage and the risk of myocardial infarction in the general population. PLoS One. 10:e0124653. DOI: 10.1371/journal.pone.0124653. PMID: 26061035. PMCID: PMC4462578.
Article56. Lo WK, Chan WW. 2013; Proton pump inhibitor use and the risk of small intestinal bacterial overgrowth: a meta-analysis. Clin Gastroenterol Hepatol. 11:483–490. DOI: 10.1016/j.cgh.2012.12.011. PMID: 23270866.
Article57. Freedberg DE, Kim LS, Yang YX. 2017; The risks and benefits of long-term use of proton pump inhibitors: expert review and best practice advice from the American Gastroenterological Association. Gastroenterology. 152:706–715. DOI: 10.1053/j.gastro.2017.01.031. PMID: 28257716.
Article58. Bolten W, Gomes JA, Stead H, Geis GS. 1992; The gastroduodenal safety and efficacy of the fixed combination of diclofenac and misoprostol in the treatment of osteoarthritis. Br J Rheumatol. 31:753–758. DOI: 10.1093/rheumatology/31.11.753. PMID: 1450797.
Article59. de Melo Gomes JA. 1992; The safety of Arthrotec in patients with rheumatoid arthritis or osteoarthritis: an assessment of the upper gastrointestinal tract by endoscopy. Scand J Rheumatol Suppl. 96:23–31. DOI: 10.3109/03009749209095096. PMID: 1439621. PMCID: PMC6509215.60. Verdickt W, Moran C, Hantzschel H, Fraga AM, Stead H, Geis GS. 1992; A double-blind comparison of the gastroduodenal safety and efficacy of diclofenac and a fixed dose combination of diclofenac and misoprostol in the treatment of rheumatoid arthritis. Scand J Rheumatol. 21:85–91. DOI: 10.3109/03009749209095074. PMID: 1570496.
Article61. Bardhan KD, Bjarnason I, Scott DL, et al. 1993; The prevention and healing of acute non-steroidal anti-inflammatory drug-associated gastroduodenal mucosal damage by misoprostol. Br J Rheumatol. 32:990–995. DOI: 10.1093/rheumatology/32.11.990. PMID: 8220939.
Article62. Graham DY, White RH, Moreland LW, et al. 1993; Duodenal and gastric ulcer prevention with misoprostol in arthritis patients taking NSAIDs. Misoprostol Study Group. Ann Intern Med. 119:257–262. DOI: 10.7326/0003-4819-119-4-199308150-00001. PMID: 8328732.63. Henriksson K, Uribe A, Sandstedt B, Nord CE. 1993; Helicobacter pylori infection, ABO blood group, and effect of misoprostol on gastroduodenal mucosa in NSAID-treated patients with rheumatoid arthritis. Dig Dis Sci. 38:1688–1696. DOI: 10.1007/BF01303179. PMID: 8359082.
Article64. Melo Gomes JA, Roth SH, Zeeh J, Bruyn GA, Woods EM, Geis GS. 1993; Double-blind comparison of efficacy and gastroduodenal safety of diclofenac/misoprostol, piroxicam, and naproxen in the treatment of osteoarthritis. Ann Rheum Dis. 52:881–885. DOI: 10.1136/ard.52.12.881. PMID: 8311540. PMCID: PMC1005220.
Article65. Roth SH, Tindall EA, Jain AK, et al. 1993; A controlled study comparing the effects of nabumetone, ibuprofen, and ibuprofen plus misoprostol on the upper gastrointestinal tract mucosa. Arch Intern Med. 153:2565–2571. DOI: 10.1001/archinte.1993.00410220073008. PMID: 8239849.
Article66. Agrawal NM, Van Kerckhove HE, Erhardt LJ, Geis GS. 1995; Misoprostol coadministered with diclofenac for prevention of gastroduodenal ulcers. A one-year study. Dig Dis Sci. 40:1125–1131. DOI: 10.1007/BF02064210. PMID: 7729275.67. Piette F, Teillet L, Naudin R, Boichut D, Capron MH. 1997; Efficacy of misoprostol in the prophylaxis of gastroduodenal lesions induced by short-term nonsteroidal antiinflammatory drug therapy in elderly patients. A multicenter double-blind, placebo-controlled trial. Rev Rhum Engl Ed. 64:259–266. PMID: 9178399.68. Bocanegra TS, Weaver AL, Tindall EA, et al. 1998; Diclofenac/misoprostol compared with diclofenac in the treatment of osteoarthritis of the knee or hip: a randomized, placebo controlled trial. Arthrotec Osteoarthritis Study Group. J Rheumatol. 25:1602–1611. DOI: 10.1002/14651858.CD004257.pub2. PMID: 9712107.69. Agrawal NM, Caldwell J, Kivitz AJ, et al. 1999; Comparison of the upper gastrointestinal safety of Arthrotec 75 and nabumetone in osteoarthritis patients at high risk for developing nonsteroidal anti-inflammatory drug-induced gastrointestinal ulcers. Clin Ther. 21:659–674. DOI: 10.1016/S0149-2918(00)88318-6. PMID: 10363732.
Article70. Rostom A, Dube C, Wells G, et al. 2002; Prevention of NSAID-induced gastroduodenal ulcers. Cochrane Database Syst Rev. (4):CD002296. DOI: 10.1002/14651858.CD002296.
Article71. Roth SH, Bennett RE, Mitchell CS, Hartman RJ. 1987; Cimetidine therapy in nonsteroidal anti-inflammatory drug gastropathy. Double-blind long-term evaluation. Arch Intern Med. 147:1798–1801. DOI: 10.1001/archinte.1987.00370100112018. PMID: 3310942.
Article72. Frank WO, Wallin BA, Berkowitz JM, et al. 1989; Reduction of indomethacin induced gastroduodenal mucosal injury and gastrointestinal symptoms with cimetidine in normal subjects. J Rheumatol. 16:1249–1252. PMID: 2810283.73. Levine LR, Cloud ML, Enas NH. 1993; Nizatidine prevents peptic ulceration in high-risk patients taking nonsteroidal anti-inflammatory drugs. Arch Intern Med. 153:2449–2454. DOI: 10.1001/archinte.1993.00410210073008. PMID: 8215749.
Article74. Taha AS, Hudson N, Hawkey CJ, et al. 1996; Famotidine for the prevention of gastric and duodenal ulcers caused by nonsteroidal antiinflammatory drugs. N Engl J Med. 334:1435–1439. DOI: 10.1056/NEJM199605303342204. PMID: 8618582.
Article75. ten Wolde S, Dijkmans BA, Janssen M, Hermans J, Lamers CB. 1996; High-dose ranitidine for the prevention of recurrent peptic ulcer disease in rheumatoid arthritis patients taking NSAIDs. Aliment Pharmacol Ther. 10:347–351. DOI: 10.1111/j.0953-0673.1996.00347.x. PMID: 8791962.
Article76. Hudson N, Taha AS, Russell RI, et al. 1997; Famotidine for healing and maintenance in nonsteroidal anti-inflammatory drug-associated gastroduodenal ulceration. Gastroenterology. 112:1817–1822. DOI: 10.1053/gast.1997.v112.pm9178671. PMID: 9178671.77. Laine L, Kivitz AJ, Bello AE, Grahn AY, Schiff MH, Taha AS. 2012; Double-blind randomized trials of single-tablet ibuprofen/high-dose famotidine vs. ibuprofen alone for reduction of gastric and duodenal ulcers. Am J Gastroenterol. 107:379–386. DOI: 10.1038/ajg.2011.443. PMID: 22186979. PMCID: PMC3321505.
Article78. Miner PB Jr, Allgood LD, Grender JM. 2007; Comparison of gastric pH with omeprazole magnesium 20.6 mg (Prilosec OTC) o.m. famotidine 10 mg (Pepcid AC) b.d. and famotidine 20 mg b.d. over 14 days of treatment. Aliment Pharmacol Ther. 25:103–109. DOI: 10.1111/j.1365-2036.2006.03129.x. PMID: 17229225.79. McRorie JW, Kirby JA, Miner PB. 2014; Histamine2-receptor antagonists: rapid development of tachyphylaxis with repeat dosing. World J Gastrointest Pharmacol Ther. 5:57–62. DOI: 10.4292/wjgpt.v5.i2.57. PMID: 24868486. PMCID: PMC4023325.
Article80. Laine L, Sloane R, Ferretti M, Cominelli F. 1995; A randomized double-blind comparison of placebo, etodolac, and naproxen on gastrointestinal injury and prostaglandin production. Gastrointest Endosc. 42:428–433. DOI: 10.1016/S0016-5107(95)70045-5. PMID: 8566633.
Article81. Roth SH, Bennett R, Caldron P, Mitchell C, Swenson C, Koepp R. 1994; A longterm endoscopic evaluation of patients with arthritis treated with nabumetone vs naproxen. J Rheumatol. 21:1118–1123. PMID: 7932425.82. Porto A, Reis C, Perdigoto R, Gonçalves M, Freitas P, Macciocchi A. 1998; Gastroduodenal tolerability of nimesulide and diclofenac in patients with osteoarthritis. Current Therapeutic Research. 59:654–665. DOI: 10.1016/S0011-393X(98)85063-7.
Article83. Emery P, Zeidler H, Kvien TK, et al. 1999; Celecoxib versus diclofenac in long-term management of rheumatoid arthritis: randomised double-blind comparison. Lancet. 354:2106–2111. DOI: 10.1016/S0140-6736(99)02332-6. PMID: 10609815.
Article84. Laine L, Harper S, Simon T, et al. 1999; A randomized trial comparing the effect of rofecoxib, a cyclooxygenase 2-specific inhibitor, with that of ibuprofen on the gastroduodenal mucosa of patients with osteoarthritis. Rofecoxib Osteoarthritis Endoscopy Study Group. Gastroenterology. 117:776–783. DOI: 10.1016/S0016-5085(99)70334-3. PMID: 10500058.85. Simon LS, Weaver AL, Graham DY, et al. 1999; Anti-inflammatory and upper gastrointestinal effects of celecoxib in rheumatoid arthritis: a randomized controlled trial. JAMA. 282:1921–1928. DOI: 10.1001/jama.282.20.1921. PMID: 10580457.86. Hawkey C, Laine L, Simon T, et al. 2000; Comparison of the effect of rofecoxib (a cyclooxygenase 2 inhibitor), ibuprofen, and placebo on the gastroduodenal mucosa of patients with osteoarthritis: a randomized, double-blind, placebo-controlled trial. The Rofecoxib Osteoarthritis Endoscopy Multinational Study Group. Arthritis Rheum. 43:370–377. DOI: 10.1002/1529-0131(200002)43:2<370::AID-ANR17>3.0.CO;2-D. PMID: 10693877.87. Hawkey CJ, Laine L, Harper SE, et al. 2001; Influence of risk factors on endoscopic and clinical ulcers in patients taking rofecoxib or ibuprofen in two randomized controlled trials. Aliment Pharmacol Ther. 15:1593–1601. DOI: 10.1046/j.1365-2036.2001.01007.x. PMID: 11563999.
Article88. Kivitz A, Eisen G, Zhao WW, Bevirt T, Recker DP. 2002; Randomized placebo-controlled trial comparing efficacy and safety of valdecoxib with naproxen in patients with osteoarthritis. J Fam Pract. 51:530–537. PMID: 12100776.89. Sikes DH, Agrawal NM, Zhao WW, Kent JD, Recker DP, Verburg KM. 2002; Incidence of gastroduodenal ulcers associated with valdecoxib compared with that of ibuprofen and diclofenac in patients with osteoarthritis. Eur J Gastroenterol Hepatol. 14:1101–1111. DOI: 10.1097/00042737-200210000-00011. PMID: 12362101.
Article90. Goldstein JL, Kivitz AJ, Verburg KM, Recker DP, Palmer RC, Kent JD. 2003; A comparison of the upper gastrointestinal mucosal effects of valdecoxib, naproxen and placebo in healthy elderly subjects. Aliment Pharmacol Ther. 18:125–132. DOI: 10.1046/j.1365-2036.2003.01650.x. PMID: 12848634.
Article91. Hawkey CJ, Laine L, Simon T, et al. 2003; Incidence of gastroduodenal ulcers in patients with rheumatoid arthritis after 12 weeks of rofecoxib, naproxen, or placebo: a multicentre, randomised, double blind study. Gut. 52:820–826. DOI: 10.1136/gut.52.6.820. PMID: 12740337. PMCID: PMC1773685.
Article92. Hunt RH, Harper S, Watson DJ, et al. 2003; The gastrointestinal safety of the COX-2 selective inhibitor etoricoxib assessed by both endoscopy and analysis of upper gastrointestinal events. Am J Gastroenterol. 98:1725–1733. DOI: 10.1111/j.1572-0241.2003.07598.x. PMID: 12907325.
Article93. Hunt RH, Harper S, Callegari P, et al. 2003; Complementary studies of the gastrointestinal safety of the cyclo-oxygenase-2-selective inhibitor etoricoxib. Aliment Pharmacol Ther. 17:201–210. DOI: 10.1046/j.1365-2036.2003.01407.x. PMID: 12534404.
Article94. Rordorf C, Kellett N, Mair S, et al. 2003; Gastroduodenal tolerability of lumiracoxib vs placebo and naproxen: a pilot endoscopic study in healthy male subjects. Aliment Pharmacol Ther. 18:533–541. DOI: 10.1046/j.1365-2036.2003.01691.x. PMID: 12950426.
Article95. Girawan D, Abdurachman SA, Djumhana A, Roslia J, Pramudiyo R. 2004; Comparison of endoscopic gastric mucosa features after administration of piroxicam to meloxicam and their correlation with dyspepsia symptoms in elderly patient with knee osteoarthritis. Acta Med Indones. 36:202–206. PMID: 15673949.96. Hawkey CC, Svoboda P, Fiedorowicz-Fabrycy IF, et al. 2004; Gastroduodenal safety and tolerability of lumiracoxib compared with Ibuprofen and celecoxib in patients with osteoarthritis. J Rheumatol. 31:1804–1810. DOI: 10.1016/s1542-3565(05)00976-6. PMID: 15338504.97. Kivitz AJ, Nayiager S, Schimansky T, Gimona A, Thurston HJ, Hawkey C. 2004; Reduced incidence of gastroduodenal ulcers associated with lumiracoxib compared with ibuprofen in patients with rheumatoid arthritis. Aliment Pharmacol Ther. 19:1189–1198. DOI: 10.1111/j.1365-2036.2004.01956.x. PMID: 15153172.
Article98. Scheiman JM, Cryer B, Kimmey MB, Rothstein RI, Riff DS, Wolfe MM. 2004; A randomized, controlled comparison of ibuprofen at the maximal over-the-counter dose compared with prescription-dose celecoxib on upper gastrointestinal mucosal injury. Clin Gastroenterol Hepatol. 2:290–295. DOI: 10.1016/S1542-3565(04)00057-6. PMID: 15067622.
Article99. Goldstein JL, Aisenberg J, Lanza F, et al. 2006; A multicenter, randomized, double-blind, active-comparator, placebo-controlled, parallel-group comparison of the incidence of endoscopic gastric and duodenal ulcer rates with valdecoxib or naproxen in healthy subjects aged 65 to 75 years. Clin Ther. 28:340–351. DOI: 10.1016/j.clinthera.2006.03.007. PMID: 16750449.
Article100. Moberly JB, Harris SI, Riff DS, et al. 2007; A randomized, double-blind, one-week study comparing effects of a novel COX-2 inhibitor and naproxen on the gastric mucosa. Dig Dis Sci. 52:442–450. DOI: 10.1007/s10620-006-9521-6. PMID: 17216336.
Article101. Sakamoto C, Kawai T, Nakamura S, Sugioka T, Tabira J. 2013; Comparison of gastroduodenal ulcer incidence in healthy Japanese subjects taking celecoxib or loxoprofen evaluated by endoscopy: a placebo-controlled, double-blind 2-week study. Aliment Pharmacol Ther. 37:346–354. DOI: 10.1111/apt.12174. PMID: 23216412.
Article102. Chan FK, Hung LC, Suen BY, et al. 2004; Celecoxib versus diclofenac plus omeprazole in high-risk arthritis patients: results of a randomized double-blind trial. Gastroenterology. 127:1038–1043. DOI: 10.1053/j.gastro.2004.07.010. PMID: 15480981.
Article103. Farkouh ME, Greenberg BP. 2009; An evidence-based review of the cardiovascular risks of nonsteroidal anti-inflammatory drugs. Am J Cardiol. 103:1227–1237. DOI: 10.1016/j.amjcard.2009.01.014. PMID: 19406264.
Article104. Chan FK, Ching JY, Suen BY, Tse YK, Wu JC, Sung JJ. 2013; Effects of Helicobacter pylori infection on long-term risk of peptic ulcer bleeding in low-dose aspirin users. Gastroenterology. 144:528–535. DOI: 10.1053/j.gastro.2012.12.038. PMID: 23333655. PMCID: PMC3607744.
Article105. Lai KC, Lam SK, Chu KM, et al. 2002; Lansoprazole for the prevention of recurrences of ulcer complications from long-term low-dose aspirin use. N Engl J Med. 346:2033–2038. DOI: 10.1056/NEJMoa012877. PMID: 12087138.
Article106. Sugano K, Matsumoto Y, Itabashi T, et al. 2011; Lansoprazole for secondary prevention of gastric or duodenal ulcers associated with long-term low-dose aspirin therapy: results of a prospective, multicenter, double-blind, randomized, double-dummy, active-controlled trial. J Gastroenterol. 46:724–735. DOI: 10.1007/s00535-011-0397-7. PMID: 21499703. PMCID: PMC3117278.
Article107. Scheiman JM, Devereaux PJ, Herlitz J, et al. 2011; Prevention of peptic ulcers with esomeprazole in patients at risk of ulcer development treated with low-dose acetylsalicylic acid: a randomised, controlled trial (OBERON). Heart. 97:797–802. DOI: 10.1136/hrt.2010.217547. PMID: 21415072. PMCID: PMC3088470.
Article108. Sanuki T, Fujita T, Kutsumi H, et al. 2012; Rabeprazole reduces the recurrence risk of peptic ulcers associated with low-dose aspirin in patients with cardiovascular or cerebrovascular disease: a prospective randomized active-controlled trial. J Gastroenterol. 47:1186–1197. DOI: 10.1007/s00535-012-0588-x. PMID: 22526273.
Article109. Sugano K, Choi MG, Lin JT, et al. 2014; Multinational, double-blind, randomised, placebo-controlled, prospective study of esomeprazole in the prevention of recurrent peptic ulcer in low-dose acetylsalicylic acid users: the LAVENDER study. Gut. 63:1061–1068. DOI: 10.1136/gutjnl-2013-304722. PMID: 24326741.
Article110. Whellan DJ, Goldstein JL, Cryer BL, et al. 2014; PA32540 (a coordinated-delivery tablet of enteric-coated aspirin 325 mg and immediate-release omeprazole 40 mg) versus enteric-coated aspirin 325 mg alone in subjects at risk for aspirin-associated gastric ulcers: results of two 6-month, phase 3 studies. Am Heart J. 168:495–502.e4. DOI: 10.1016/j.ahj.2014.05.017. PMID: 25262259.
Article111. Iwakiri R, Higuchi K, Kato M, et al. 2014; Randomised clinical trial: prevention of recurrence of peptic ulcers by rabeprazole in patients taking low-dose aspirin. Aliment Pharmacol Ther. 40:780–795. DOI: 10.1111/apt.12907. PMID: 25100080.
Article112. Sung JJ, Lau JY, Ching JY, et al. 2010; Continuation of low-dose aspirin therapy in peptic ulcer bleeding: a randomized trial. Ann Intern Med. 152:1–9. DOI: 10.7326/0003-4819-152-1-201001050-00179. PMID: 19949136.113. Acosta RD, Abraham NS, et al. ASGE Standards of Practice Committee. 2016; The management of antithrombotic agents for patients undergoing GI endoscopy. Gastrointest Endosc. 83:3–16. DOI: 10.1016/j.gie.2015.09.035. PMID: 26621548. PMCID: PMC7478743.
Article114. Gralnek IM, Dumonceau JM, Kuipers EJ, et al. 2015; Diagnosis and management of nonvariceal upper gastrointestinal hemorrhage: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 47:a1–a46. DOI: 10.1055/s-0034-1393172. PMID: 26417980.
Article115. Chan FKL, Goh KL, Reddy N, et al. 2018; Management of patients on antithrombotic agents undergoing emergency and elective endoscopy: joint Asian Pacific Association of Gastroenterology (APAGE) and Asian Pacific Society for Digestive Endoscopy (APSDE) practice guidelines. Gut. 67:405–417. DOI: 10.1136/gutjnl-2017-315131. PMID: 29331946. PMCID: PMC5868286.
Article116. Witt DM, Delate T, Garcia DA, et al. 2012; Risk of thromboembolism, recurrent hemorrhage, and death after warfarin therapy interruption for gastrointestinal tract bleeding. Arch Intern Med. 172:1484–1491. DOI: 10.1001/archinternmed.2012.4261. PMID: 22987143.
Article117. Lee JK, Kang HW, Kim SG, Kim JS, Jung HC. 2012; Risks related with withholding and resuming anticoagulation in patients with non-variceal upper gastrointestinal bleeding while on warfarin therapy. Int J Clin Pract. 66:64–68. DOI: 10.1111/j.1742-1241.2011.02827.x. PMID: 22171905.
Article118. Qureshi W, Mittal C, Patsias I, et al. 2014; Restarting anticoagulation and outcomes after major gastrointestinal bleeding in atrial fibrillation. Am J Cardiol. 113:662–668. DOI: 10.1016/j.amjcard.2013.10.044. PMID: 24355310. PMCID: PMC4646074.
Article119. Baigent C, Blackwell L, et al. Antithrombotic Trialists' (ATT) Collaboration. 2009; Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 373:1849–1860. DOI: 10.1016/S0140-6736(09)60503-1. PMID: 19482214. PMCID: PMC2715005.120. Pearce LA, Hart RG, Halperin JL. 2000; Assessment of three schemes for stratifying stroke risk in patients with nonvalvular atrial fibrillation. Am J Med. 109:45–51. DOI: 10.1016/S0002-9343(00)00440-X. PMID: 10936477.
Article121. Veitch AM, Vanbiervliet G, Gershlick AH, et al. 2016; Endoscopy in patients on antiplatelet or anticoagulant therapy, including direct oral anticoagulants: British Society of Gastroenterology (BSG) and European Society of Gastrointestinal Endoscopy (ESGE) guidelines. Endoscopy. 48:c1. DOI: 10.1055/s-0042-122686. PMID: 28114689.
Article122. Douketis JD, Spyropoulos AC, Spencer FA, et al. 2012; Perioperative management of antithrombotic therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 141(Suppl 2):e326S–e350S. DOI: 10.1378/chest.11-2298. PMID: 22315266. PMCID: PMC3278059.123. Hawn MT, Graham LA, Richman JS, Itani KM, Henderson WG, Maddox TM. 2013; Risk of major adverse cardiac events following noncardiac surgery in patients with coronary stents. JAMA. 310:1462–1472. DOI: 10.1001/jama.2013.278787. PMID: 24101118.
Article124. Lanas A, García-Rodríguez LA, Arroyo MT, et al. 2007; Effect of antisecretory drugs and nitrates on the risk of ulcer bleeding associated with nonsteroidal anti-inflammatory drugs, antiplatelet agents, and anticoagulants. Am J Gastroenterol. 102:507–515. DOI: 10.1111/j.1572-0241.2006.01062.x. PMID: 17338735.
Article125. Massó González EL, García Rodríguez LA. 2008; Proton pump inhibitors reduce the long-term risk of recurrent upper gastrointestinal bleeding: an observational study. Aliment Pharmacol Ther. 28:629–637. DOI: 10.1111/j.1365-2036.2008.03780.x. PMID: 18616644.126. Lin KJ, Hernández-Díaz S, García Rodríguez LA. 2011; Acid suppressants reduce risk of gastrointestinal bleeding in patients on antithrombotic or anti-inflammatory therapy. Gastroenterology. 141:71–79. DOI: 10.1053/j.gastro.2011.03.049. PMID: 21458456.
Article127. Lauffenburger JC, Rhoney DH, Farley JF, Gehi AK, Fang G. 2015; Predictors of gastrointestinal bleeding among patients with atrial fibrillation after initiating dabigatran therapy. Pharmacotherapy. 35:560–568. DOI: 10.1002/phar.1597. PMID: 26044889. PMCID: PMC4750642.
Article128. Chan EW, Lau WC, Leung WK, et al. 2015; Prevention of dabigatran-related gastrointestinal bleeding with gastroprotective agents: a population-based study. Gastroenterology. 149:586–95.e3. DOI: 10.1053/j.gastro.2015.05.002. PMID: 25960019.
Article129. Ray WA, Chung CP, Murray KT, et al. 2016; Association of proton pump inhibitors with reduced risk of warfarin-related serious upper gastrointestinal bleeding. Gastroenterology. 151:1105–1112.e10. DOI: 10.1053/j.gastro.2016.08.054. PMID: 27639805. PMCID: PMC5124401.130. Sugano K. 2015; How do we manage serious gastrointestinal adverse events associated with anti-thrombotic therapy? Expert Rev Gastroenterol Hepatol. 9:5–8. DOI: 10.1586/17474124.2014.945913. PMID: 25096360. PMCID: PMC6394924.
Article131. Agewall S, Cattaneo M, Collet JP, et al. 2013; Expert position paper on the use of proton pump inhibitors in patients with cardiovascular disease and antithrombotic therapy. Eur Heart J. 34:1708–1713b. DOI: 10.1093/eurheartj/eht042. PMID: 23425521.
Article132. Teichert M, van Noord C, Uitterlinden AG, et al. 2011; Proton pump inhibitors and the risk of overanticoagulation during acenocoumarol maintenance treatment. Br J Haematol. 153:379–385. DOI: 10.1111/j.1365-2141.2011.08633.x. PMID: 21418179.
Article133. Hata M, Shiono M, Akiyama K, et al. 2015; Incidence of drug interaction when using proton pump inhibitor and warfarin according to cytochrome P450 2C19 (CYP2C19) genotype in Japanese. Thorac Cardiovasc Surg. 63:45–50. DOI: 10.1055/s-0034-1383814. PMID: 25068772.
Article134. EINSTEIN Investigators, Bauersachs R, Berkowitz SD, et al. 2010; Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 363:2499–2510. DOI: 10.1056/NEJMoa1007903. PMID: 21128814.
Article135. Granger CB, Alexander JH, McMurray JJ, et al. 2011; Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 365:981–992. DOI: 10.1056/NEJMoa1107039. PMID: 21870978.136. Kuwayama T, Osanai H, Ajioka M, et al. 2017; Influence of proton pump inhibitors on blood dabigatran concentrations in Japanese patients with non-valvular atrial fibrillation. J Arrhythm. 33:619–623. DOI: 10.1016/j.joa.2017.07.013. PMID: 29255511. PMCID: PMC5729000.
Article137. Bolek T, Samoš M, Stančiaková L, et al. 2019; The impact of proton pump inhibition on dabigatran levels in patients with atrial fibrillation. Am J Ther. 26:e308–e313. DOI: 10.1097/MJT.0000000000000599. PMID: 28452843.
Article138. Bolek T, Samoš M, Škorňová I, et al. 2018; Proton pump inhibition in patients treated with novel antithrombotic drugs: should we worry about thrombosis? J Cardiovasc Pharmacol. 72:71–76. DOI: 10.1097/FJC.0000000000000593. PMID: 29738377.139. Hyun KR, Kang S, Lee S. 2016; Population aging and healthcare expenditure in Korea. Health Econ. 25:1239–1251. DOI: 10.1002/hec.3209. PMID: 26085120. PMCID: PMC7473975.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Management of Peptic Ulcer Bleeding in Patients Taking Aspirin or Anticoagulant
- Prevention of Peptic Ulcer Associated with Aspirin and Antiplatelet Agent
- Clinical Guidelines for Drug-Related Peptic Ulcer, 2020 Revised Edition
- Helicobacter pylori Eradication in Drug-related Peptic Ulcer
- A clinical review of the 188 cases of peptic ulcer perforations