Ann Surg Treat Res.
2020 Oct;99(4):238-246. 10.4174/astr.2020.99.4.238.
Comparison of outcome between liver resection, radiofrequency ablation, and transarterial therapy for multiple small hepatocellular carcinoma within the Milan criteria
- Affiliations
-
- 1Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 2Department of Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 3Department of Radiology and Center for Imaging Science, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- KMID: 2506655
- DOI: http://doi.org/10.4174/astr.2020.99.4.238
Abstract
- Purpose
Although surgical resection is usually considered for a single tumor, several reports have suggested that resection can be considered for multiple tumors. The objective of this study was to determine whether resection could provide better long-term outcome for patients with multiple hepatocellular carcinomas (HCCs) within Milan criteria.
Methods
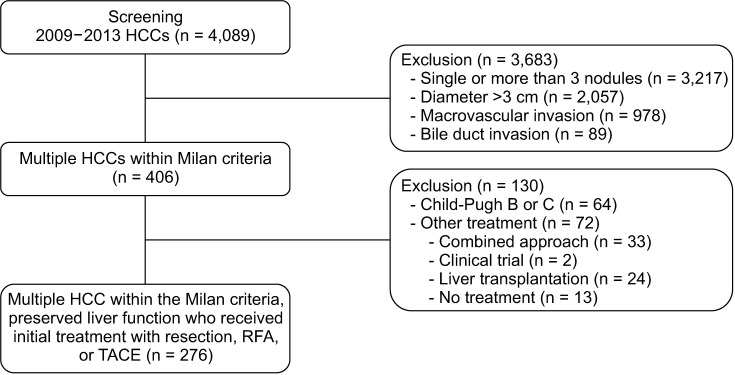
A total of 276 patients with multiple HCCs within Milan criteria with liver function preserved who underwent resection, radiofrequency ablation (RFA), or transarterial chemoembolization (TACE) between 2009 and 2013 were analyzed. Propensity-score (PS) matching was conducted.
Results
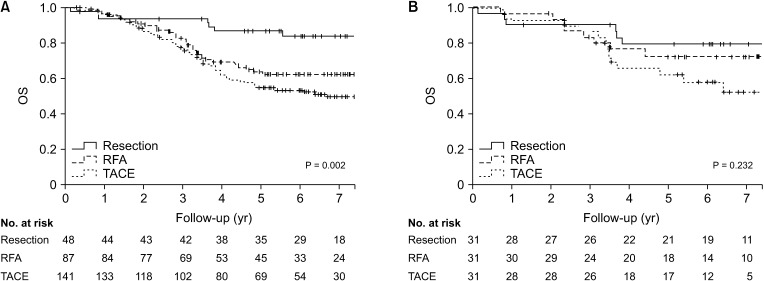
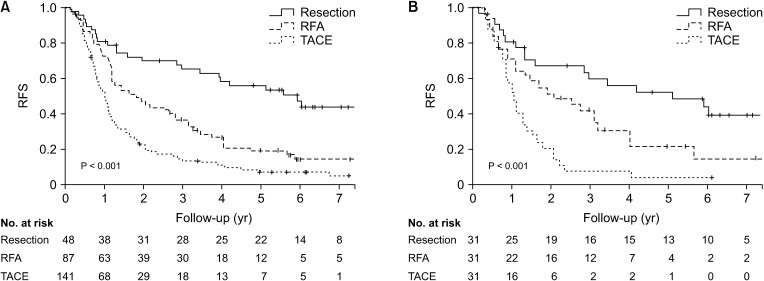
Five-year overall survival (OS) and recurrence-free survival (RFS) were better in the resection group than that in the RFA or TACE group. Patients who underwent resection had more preserved liver function and different tumor characteristics compared to those received RFA or TACE. With similar baseline characteristics generated in the PS model, there was no difference in 5-year OS among 3 groups (79.5% vs. 72.3% or 62.0%, P = 0.232), but the 5-year RFS was better for patients who received resection than those who received RFA or TACE (51.9% vs. 22.0% or 0.0%, P < 0.001). Although the major complication rate was slightly higher than RFA or TACE, there was no significant difference between the 3 groups before and after PS matching.
Conclusion
Resection was associated with better RFS than RFA or TACE and showed comparable OS in multiple HCC patients within the Milan criteria, but at a cost of slightly increased risk of complication. Resection can be considered as a first-line option if selected appropriately.
Keyword
Figure
Cited by 2 articles
-
2022 KLCA-NCC Korea practice guidelines for the management of hepatocellular carcinoma
J Liver Cancer. 2023;23(1):1-120. doi: 10.17998/jlc.2022.11.07.Exploring the role of liver resection as a first-line treatment option for multinodular BCLC-A hepatocellular carcinoma
Joo Hyun Oh, Dong Hyun Sinn
J Liver Cancer. 2024;24(2):126-128. doi: 10.17998/jlc.2024.08.08.
Reference
-
1. Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003; 362:1907–1917. PMID: 14667750.2. Wada H, Eguchi H, Noda T, Ogawa H, Yamada D, Tomimaru Y, et al. Selection criteria for hepatic resection in intermediate-stage (BCLC stage B) multiple hepatocellular carcinoma. Surgery. 2016; 160:1227–1235. PMID: 27395761.3. European Association for the Study of the Liver. European Organisation for Research and Treatment of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012; 56:908–943. PMID: 22424438.4. Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018; 67:358–380. PMID: 28130846.5. Korean Liver Cancer Study Group (KLCSG). National Cancer Center, Korea (NCC). 2014 KLCSG-NCC Korea practice guideline for the management of hepatocellular carcinoma. Gut Liver. 2015; 9:267–317. PMID: 25918260.6. Manns MP, Czaja AJ, Gorham JD, Krawitt EL, Mieli-Vergani G, Vergani D, et al. Diagnosis and management of autoimmune hepatitis. Hepatology. 2010; 51:2193–2213. PMID: 20513004.7. Jiang L, Yan L, Wen T, Li B, Zeng Y, Yang J, et al. Comparison of outcomes of hepatic resection and radiofrequency ablation for hepatocellular carcinoma patients with multifocal tumors meeting the Barcelona-Clinic Liver Cancer stage A classification. J Am Coll Surg. 2015; 221:951–961. PMID: 26362135.8. Ishizawa T, Hasegawa K, Aoki T, Takahashi M, Inoue Y, Sano K, et al. Neither multiple tumors nor portal hypertension are surgical contraindications for hepatocellular carcinoma. Gastroenterology. 2008; 134:1908–1916. PMID: 18549877.9. Yi HM, Zhang W, Ai X, Li KY, Deng YB. Radiofrequency ablation versus surgical resection for the treatment of hepatocellular carcinoma conforming to the Milan criteria: systemic review and meta-analysis. Int J Clin Exp Med. 2014; 7:3150–3163. PMID: 25419346.10. Li L, Zhang J, Liu X, Li X, Jiao B, Kang T. Clinical outcomes of radiofrequency ablation and surgical resection for small hepatocellular carcinoma: a meta-analysis. J Gastroenterol Hepatol. 2012; 27:51–58. PMID: 22004366.11. Huang J, Yan L, Cheng Z, Wu H, Du L, Wang J, et al. A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan criteria. Ann Surg. 2010; 252:903–912. PMID: 21107100.12. Duan C, Liu M, Zhang Z, Ma K, Bie P. Radiofrequency ablation versus hepatic resection for the treatment of early-stage hepatocellular carcinoma meeting Milan criteria: a systematic review and meta-analysis. World J Surg Oncol. 2013; 11:190. PMID: 23941614.13. Wang Y, Luo Q, Li Y, Deng S, Wei S, Li X. Radiofrequency ablation versus hepatic resection for small hepatocellular carcinomas: a meta-analysis of randomized and nonrandomized controlled trials. PLoS One. 2014; 9:e84484. PMID: 24404166.14. Lü MD, Kuang M, Liang LJ, Xie XY, Peng BG, Liu GJ, et al. [Surgical resection versus percutaneous thermal ablation for early-stage hepatocellular carcinoma: a randomized clinical trial]. Zhonghua Yi Xue Za Zhi. 2006; 86:801–805. PMID: 16681964.15. Feng K, Yan J, Li X, Xia F, Ma K, Wang S, et al. A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J Hepatol. 2012; 57:794–802. PMID: 22634125.16. Cucchetti A, Piscaglia F, Cescon M, Colecchia A, Ercolani G, Bolondi L, et al. Cost-effectiveness of hepatic resection versus percutaneous radiofrequency ablation for early hepatocellular carcinoma. J Hepatol. 2013; 59:300–307. PMID: 23603669.17. Farinati F, Sergio A, Baldan A, Giacomin A, Di Nolfo MA, Del Poggio P, et al. Early and very early hepatocellular carcinoma: when and how much do staging and choice of treatment really matter? A multi-center study. BMC Cancer. 2009; 9:33. PMID: 19171074.18. Ishikawa K, Chiba T, Ooka Y, Suzuki E, Ogasawara S, Maeda T, et al. Transarterial chemoembolization as a substitute to radiofrequency ablation for treating Barcelona Clinic Liver Cancer stage 0/A hepatocellular carcinoma. Oncotarget. 2018; 9:21560–21568. PMID: 29765560.19. Choi SH, Choi GH, Kim SU, Park JY, Joo DJ, Ju MK, et al. Role of surgical resection for multiple hepatocellular carcinomas. World J Gastroenterol. 2013; 19:366–374. PMID: 23372359.20. Poon RT, Fan ST, Lo CM, Liu CL, Wong J. Long-term survival and pattern of recurrence after resection of small hepatocellular carcinoma in patients with preserved liver function: implications for a strategy of salvage transplantation. Ann Surg. 2002; 235:373–382. PMID: 11882759.21. Johnson PJ, Berhane S, Kagebayashi C, Satomura S, Teng M, Reeves HL, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol. 2015; 33:550–558. PMID: 25512453.22. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004; 240:205–213. PMID: 15273542.23. Rassen JA, Shelat AA, Franklin JM, Glynn RJ, Solomon DH, Schneeweiss S. Matching by propensity score in cohort studies with three treatment groups. Epidemiology. 2013; 24:401–409. PMID: 23532053.24. Cho YK, Kim JK, Kim WT, Chung JW. Hepatic resection versus radiofrequency ablation for very early stage hepatocellular carcinoma: a Markov model analysis. Hepatology. 2010; 51:1284–1290. PMID: 20099299.25. Yin L, Li H, Li AJ, Lau WY, Pan ZY, Lai EC, et al. Partial hepatectomy vs. transcatheter arterial chemoembolization for resectable multiple hepatocellular carcinoma beyond Milan Criteria: a RCT. J Hepatol. 2014; 61:82–88. PMID: 24650695.26. Kim JY, Sinn DH, Gwak GY, Choi GS, Saleh AM, Joh JW, et al. Transarterial chemoembolization versus resection for intermediate-stage (BCLC B) hepatocellular carcinoma. Clin Mol Hepatol. 2016; 22:250–258. PMID: 27377909.27. Xu Q, Kobayashi S, Ye X, Meng X. Comparison of hepatic resection and radiofrequency ablation for small hepatocellular carcinoma: a meta-analysis of 16,103 patients. Sci Rep. 2014; 4:7252. PMID: 25429732.28. Wang YY, Zhong JH, Su ZY, Huang JF, Lu SD, Xiang BD, et al. Albumin-bilirubin versus Child-Pugh score as a predictor of outcome after liver resection for hepatocellular carcinoma. Br J Surg. 2016; 103:725–734. PMID: 27005482.29. Toyoda H, Lai PB, O'Beirne J, Chong CC, Berhane S, Reeves H, et al. Long-term impact of liver function on curative therapy for hepatocellular carcinoma: application of the ALBI grade. Br J Cancer. 2016; 114:744–750. PMID: 27022825.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Role of Combination of Transarterial Chemoebolization and Radiofrequency Ablation for Hepatocellular Carcinoma Treatment
- Efficacy of Hepatic Arterial Infusion Chemotherapy and Radiofrequency Ablation against Hepatocellular Carcinoma Refractory to Transarterial Chemoembolization and Vascular Variation: A Case Study
- Current status and future of radiofrequency ablation for hepatocellular carcinoma
- Radiofrequency Thermal Ablation of Hepatocellular Carcinomas
- Outcome of Hepatic Resection for Hepatocellular Carcinoma within the Milan Criteria in Child-Pugh Class A Patients