Korean J Radiol.
2000 Dec;1(4):175-184. 10.3348/kjr.2000.1.4.175.
Radiofrequency Thermal Ablation of Hepatocellular Carcinomas
- Affiliations
-
- 1Department of Radiology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. hklim@smc.samsung.co.kr
- KMID: 1378942
- DOI: http://doi.org/10.3348/kjr.2000.1.4.175
Abstract
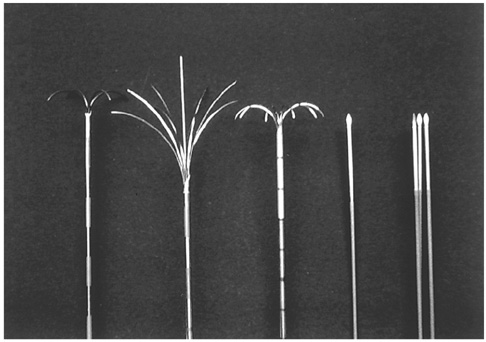
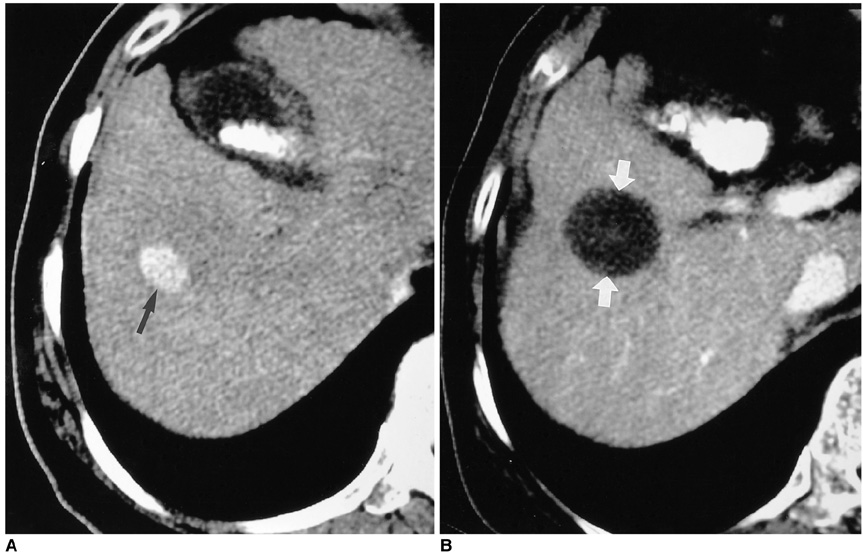
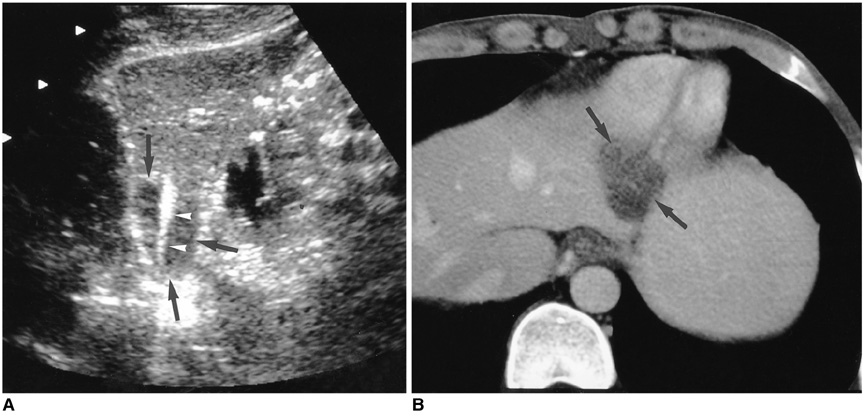
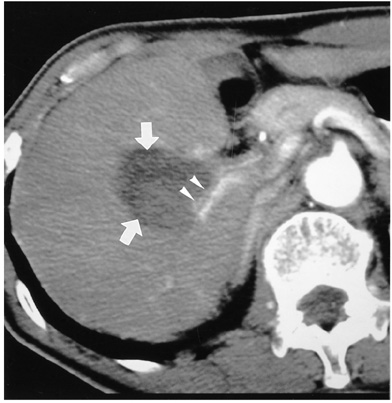
- Although surgical resection remains the best option as potentially curative therapy for hepatocellular carcinoma, radiofrequency thermal ablation has begun to receive much attention as an effective minimally invasive technique for the local control of unresectable malignant hepatic tumors. Most recent radiofrequency devices equipped with a powerful generator and larger needle electrode permit larger thermal lesions, up to 5 cm in diameter, with a single ablation. In this article, the author reviews the technical developments and early clinical results obtained with radiofrequency ablation techniques.
MeSH Terms
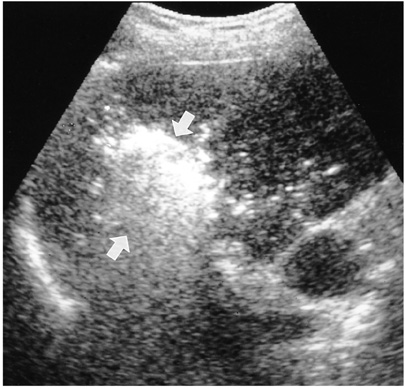
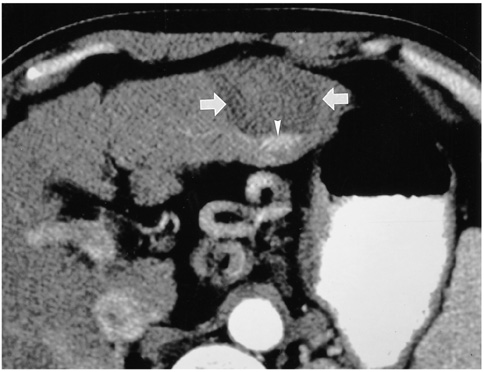
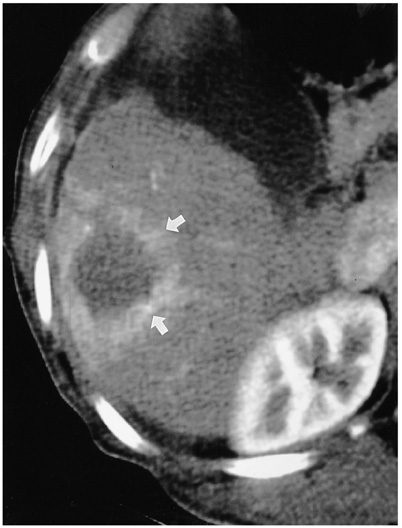
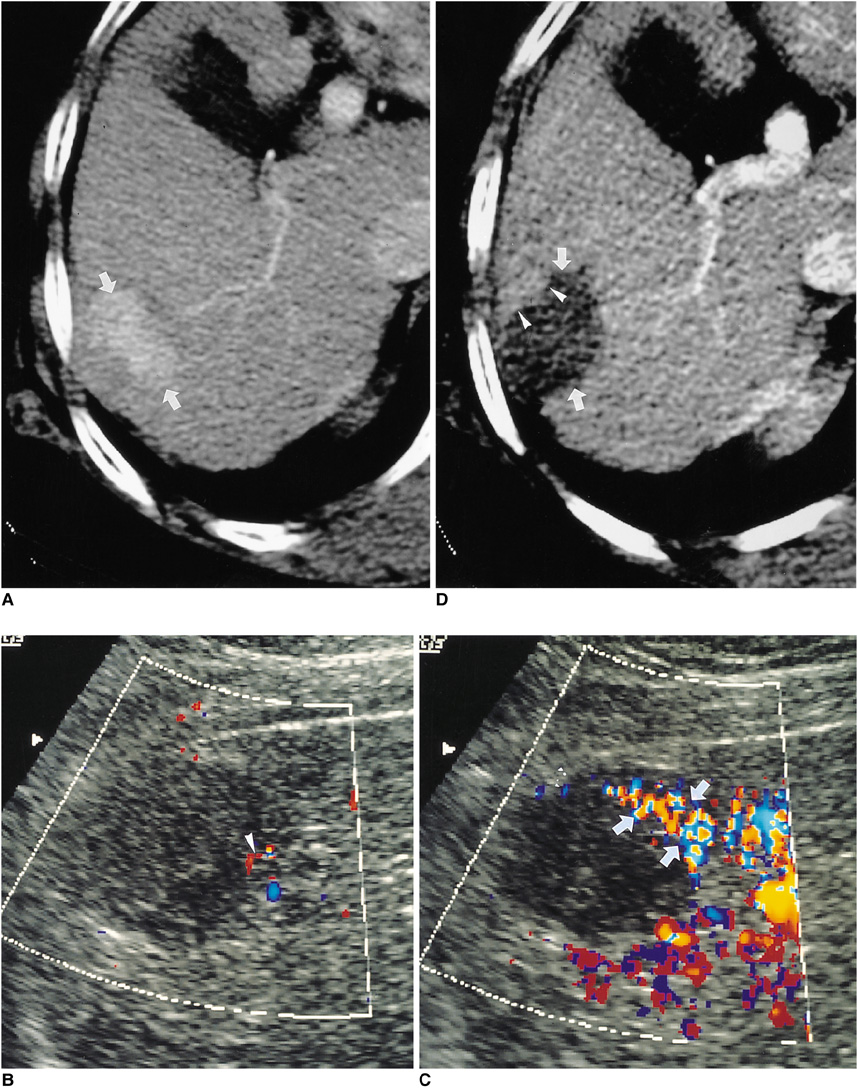
Figure
Reference
-
1. Colombo M. Hepatocellular carcinoma. J Hepatol. 1992. 15:225–236.2. Tsuzuki T, Sugioka A, Ueda M. Hepatic resection for hepatocellular carcinoma. Surgery. 1990. 107:511–520.3. Liver Cancer Study Group of Japan. Primary liver cancer in Japan: clinicopathologic features and results of surgical treatment. Ann Surg. 1990. 211:277–284.4. Farmer DG, Rosove MH, Shaked A, Busuttil RW. Current treatment modalities for hepatocellular carcinoma. Ann Surg. 1994. 219:236–247.5. Livraghi T, Bolondi L, Lazzaroni S, et al. Percutaneous ethanol injection in the treatment of hepatocellular carcinoma in cirrhosis: a study in 207 patients. Cancer. 1992. 69:925–929.6. Honda N, Guo Q, Uchida H, Ohishi H, Hiasa Y. Percutaneous hot saline injection therapy for hepatic tumors: an alternative to percutaneous ethanol injection therapy. Radiology. 1994. 190:53–57.7. Amin Z, Donald JJ, Masters A, et al. Hepatic metastases: interstitial laser photocoagulation with real-time sonography monitoring and dynamic CT evaluation of treatment. Radiology. 1993. 187:339–347.8. Murakami R, Yoshimatsu S, Yamashita Y, Matsukawa T, Takahashi M, Sagara K. Treatment of hepatocellular carcinoma: value of percutaneous microwave coagulation. AJR. 1995. 164:1159–1164.9. McGahan JP, Browning PD, Brock JM, Tesluk H. Hepatic ablation using radiofrequency electrocautery. Invest Radiol. 1990. 25:264–270.10. Goldberg SN, Gazelle GS, Dawson SI, et al. Tissue ablation with radiofrequency using multiprobe arrays. Acad Radiol. 1995. 2:670–674.11. Livraghi T, Goldberg SN, Monti F, et al. Saline-enhanced radiofrequency tissue ablation in the treatment of liver metastasis. Radiology. 1997. 202:205–210.12. Rossi S, Buscarini E, Garbagnati F, et al. Percutaneous treatment of small hepatic tumors by an expandable RF needle electrode. AJR. 1998. 170:1015–1022.13. Curley SA, Izzo F, Delrio P, et al. Radiofrequency ablation of unresectable primary and metastatic hepatic malignancies: results in 123 patients. Ann Surg. 1999. 230:1–8.14. Solbiati L, Goldberg SN, Ierace T, et al. Hepatic metastases: percutaneous radio-frequency ablation with cooled-tip electrodes. Radiology. 1997. 205:367–373.15. Rossi S, Di Stasi M, Buscarini E, et al. Percutaneous RF interstitial thermal ablation in the treatment of hepatic cancer. AJR. 1996. 167:759–768.16. Livraghi T, Goldberg SN, Lazzaroni S, Meloni F, Solbiati L, Gazelle GS. Small hepatocellular carcinoma: treatment with radio-frequency ablation versus ethanol injection. Radiology. 1999. 210:655–661.17. Livraghi T, Goldberg SN, Lazzaroni S, et al. Hepatocellular carcinoma: radio-frequency ablation of medium and large lesions. Radiology. 2000. 214:761–768.18. Goldberg SN, Solbiati L, Hahn PF. Large-volume tissue ablation with radiofrequency by using a clustered, internally cooled electrode: laboratory and clinical experience in liver metastases. Radiology. 1998. 209:371–379.19. Choi D, Lim HK, Park JM, et al. An experimental study on hepatic ablation using an expandable radiofrequency needle electrode. J of Korean Radiol Soc. 1999. 41:1127–1132.20. Choi D, Lim HK, Kim SH, et al. Radiofrequency ablation of small heaptocellular carcinoma: early experience of efficacy and safety. J of Korean Radiol Soc. 2000. 42:743–749.21. Hornback NB. Historical aspects of hyperthermia in cancer therapy. Radiol Clin North Am. 1989. 27:481–488.22. Coley WB. The treatment of malignant tumors by repeated innoculations of erysipelas, with a report of ten original cases. Am J Med Sci. 1893. 105:487–492.23. Rosomoff Hl, Carroll F, Brown J, Sheptak T. Percutaneous radiofrequency cervical cordotomy technique. J Neurosurgery. 1965. 23:639–644.24. LeVenn HH, Ahmed N, Piccone VA, Shugaar S, Falk G. Radiofrequency therapy: clinical experience. Ann N Y Acad Sci. 1980. 335:362–371.25. Seki T, Wakabayashi M, Nakagawa T, et al. Ultrasonically guided percutaneous microwave coagulation therapy for small hepatocellular carcinoma. Cancer. 1994. 74:817–825.26. Dickson JA, Calderwood SK. Temperature range and selective sensitivity of tumors to hyperthermia: a critical review. Ann N Y Acad Sci. 1980. 335:180–205.27. Hill RP, Hunt JW. Tannock IF, Hill RF, editors. Hyperthermia. The Basic Science of Oncology. 1987. New York: Pergamon Press;337–357.28. Allain JC, LeLouis M, Bailey AJ, Delaunay A. Isometric tension developed during heating of collagen tissues: relationship with collagen cross-linking. Biochimica et Biophysica Acta. 1978. 533:147–155.29. Haines DE, Verow AF. Observations on the electrode-tissue interface temperature and effect on electrical impendance during radiofrequency ablation of ventricular myocardium. Circ. 1990. 82:1034–1038.30. Goldberg SN, Hahn PF, Tanabe KK, et al. Percutaneous radiofrequency tissue ablation: does perfusion-mediated tissue cooling limit coagulation necrosis? J Vasc Interv Radiol. 1998. 9:101–111.31. Patterson EJ, Scudamore CH, Owen DA, Nagy AG, Buczkowski AK. Radiofrequency ablation of porcine liver in vivo: effects of blood flow and treatement time on lesion size. Annals of Surgery. 1998. 227:559–565.32. Goldberg SN, Hahn PF, Halpern EF, Fogle RM, Gazelle GS. Radio-frequency tissue ablation: effect of pharmacologic modulation of blood flow on coagulation diameter. Radiology. 1998. 209:761–767.33. Goldberg SN, Gazelle GS, Solbiati L, et al. Ablation of liver tumors using percutaneous RF therapy. AJR. 1998. 170:1023–1028.34. Choi D, Lim HK, Lee WJ, et al. Evaluation of therapeutic response in hepatocellular carcinoma treated with percutaneous radio-frequency ablation: usefulness of power Doppler US with a microbubble contrast agent-preliminary results. Radiology. 2000. 217:558–563.35. Lim HK. Radiofrequency ablation of hepatocellular carcinoma. Proceedings of the 4th Asian Pacific Congress of Cardiovascular and Interventional Radiology. 2000. 120–122.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Completely Ablated Hepatocellular Carcinoma by Percutaneous Radiofrequency Thermal Ablation
- Skin Burn after Laparoscopic Radiofrequency Thermal Ablation for Uterine Myoma : A case report
- Radiofrequency Thermal Ablation of Hepatocellular Carcinoma
- Microwave thermosphere versus radiofrequency ablation for hepatocellular carcinoma: Are we approaching the time to end the debate?
- Chemoembolization combined with radiofrequency ablation is the best option for the local treatment of early hepatocellular carcinoma?