Obstet Gynecol Sci.
2020 Sep;63(5):594-604. 10.5468/ogs.20073.
Isolation of mesenchymal stem cells from Pap smear samples
- Affiliations
-
- 1Department of Biotechnology, College of Life Sciences and Biotechnology, Korea University, Seoul, Korea
- 2Department of Obstetrics and Gynecology, College of Medicine, Korea University, Seoul, Korea
- 3Institute of Animal Molecular Biotechnology, Korea University, Seoul, Korea
- KMID: 2506502
- DOI: http://doi.org/10.5468/ogs.20073
Abstract
Objective
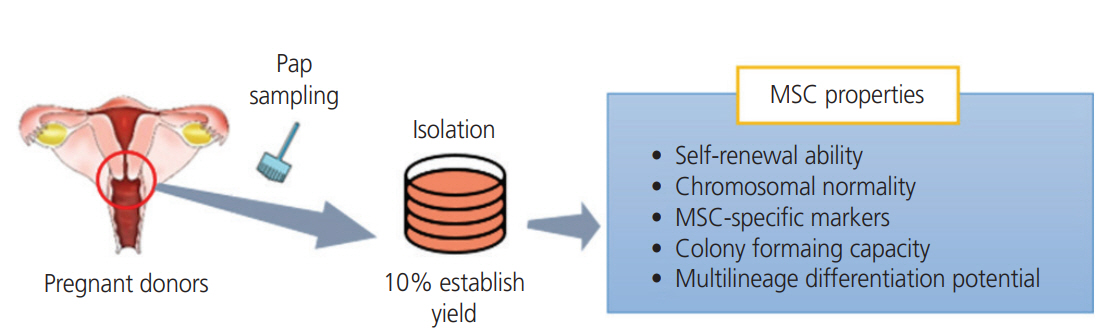
Exploiting their ability to differentiate into mesenchymal lineages like cartilage, bone, fat, and muscle, and to elicit paracrine effects, mesenchymal stem cells (MSCs) are widely used in clinical settings to treat tissue injuries and autoimmune disorders. One of accessible sources of MSC is the samples used for Papanicolaou (Pap) test, which is a cervical screening method for detecting potentially pre-cancerous and cancerous alterations in the cervical cells and to diagnose genetic abnormalities in fetuses. This study aimed to identify and isolate the stem cells from Pap smear samples collected from pregnant women, and to trace the origin of these cells to maternal or fetal tissue, and characterize their stem cell properties.
Methods
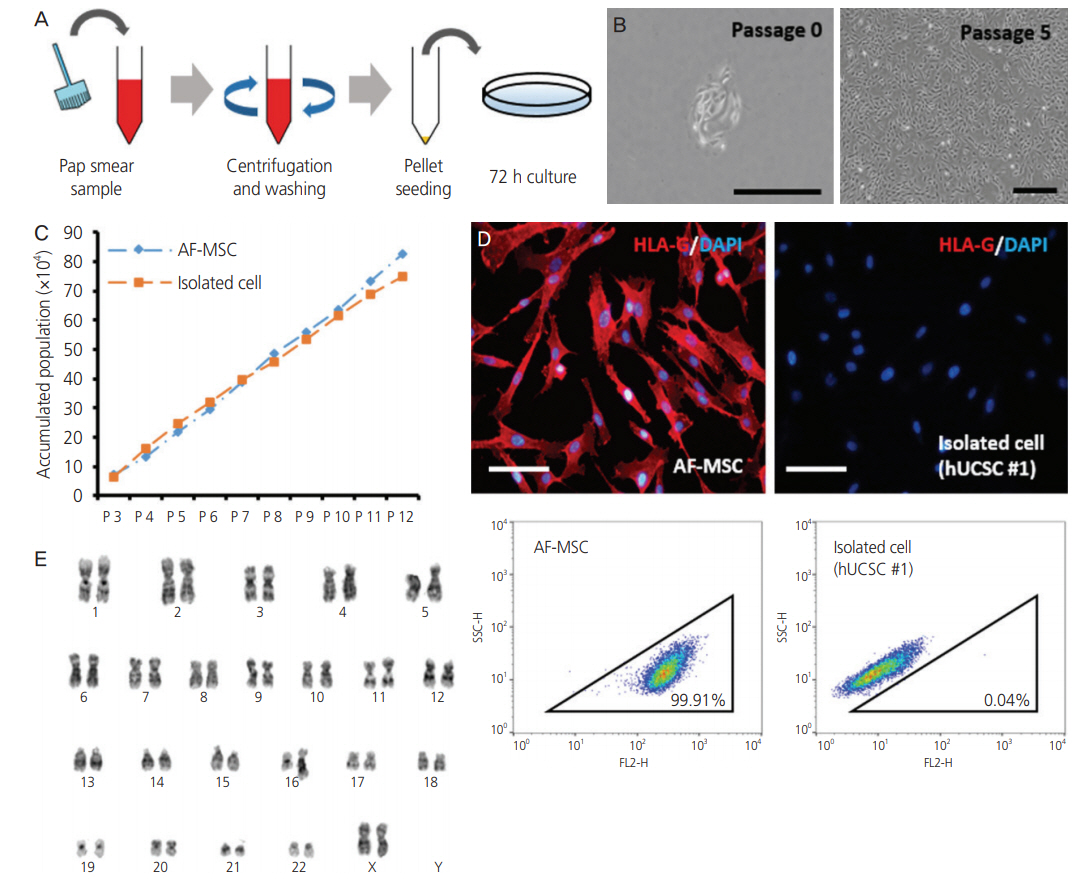
To investigate the possibility and efficiency of establishing MSC lines from the Pap smear samples, we were able to establish 6 cell lines from Pap smear samples from 60 pregnant women at different stages of gestation.
Results
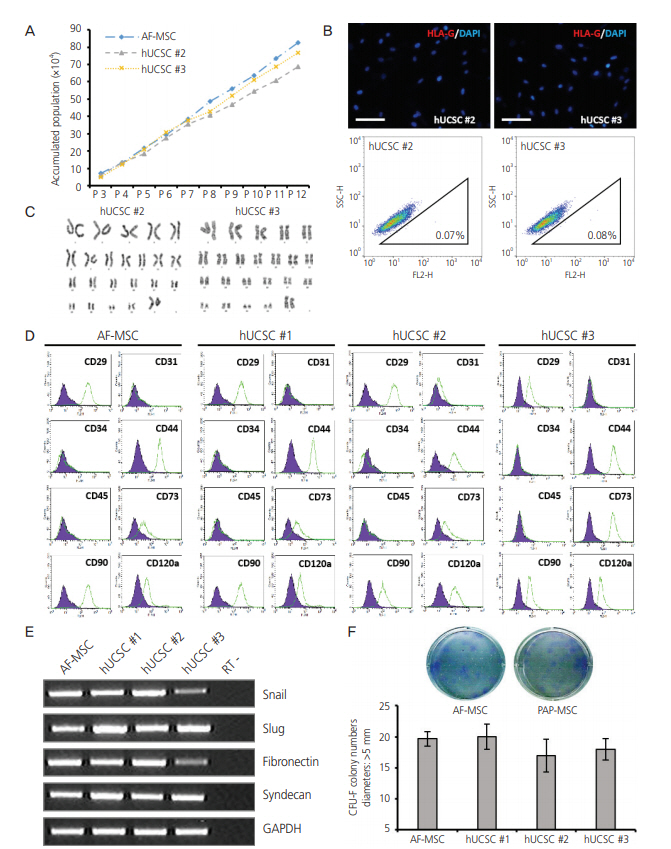
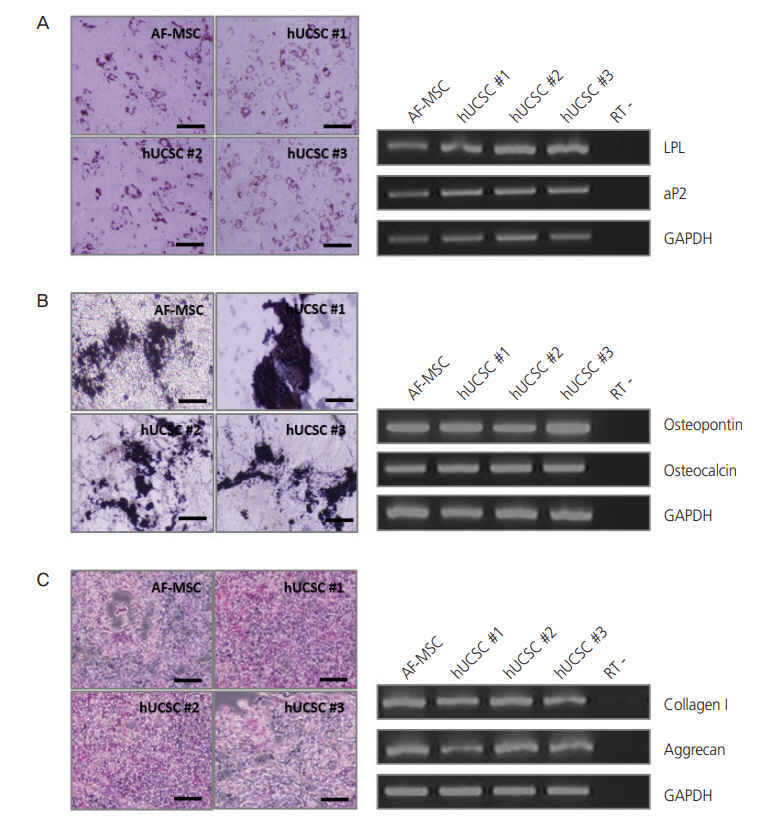
The 3 cell lines randomly selected among the 6 established in this study, displayed high proliferation rates, several characteristics of MSCs, and the capacity to differentiate into adipocytes, osteocytes, and chondrocytes. Our study identified that the stem cell lines obtainable from Pap smear sampling were uterine cervical stromal cells (UCSCs) and had 10% efficiency of establishment.
Conclusion
Despite their low efficiency of establishment, human UCSCs from Pap smear samples can become a simple, safe, low-cost, and donor-specific source of MSCs for stem cell therapy and regenerative medicine.
Figure
Reference
-
References
1. Squillaro T, Peluso G, Galderisi U. Clinical trials with mesenchymal stem cells: an update. Cell Transplant. 2016; 25:829–48.
Article2. Hwang IS, Kim HG, Jo DH, Lee DH, Koo YH, Song YJ, et al. Comparison with human amniotic membrane- and adipose tissue-derived mesenchymal stem cells. Korean J Obstet Gynecol. 2011; 54:674–83.
Article3. Wu M, Zhang R, Zou Q, Chen Y, Zhou M, Li X, et al. Comparison of the biological characteristics of mesenchymal stem cells derived from the human placenta and umbilical cord. Sci Rep. 2018; 8:5014.
Article4. Papanicolaou GN. A new procedure for staining vaginal smears. Science. 1942; 95:438–9.
Article5. Pfeifer I, Benachi A, Saker A, Bonnefont JP, Mouawia H, Broncy L, et al. Cervical trophoblasts for non-invasive single-cell genotyping and prenatal diagnosis. Placenta. 2016; 37:56–60.
Article6. Eiró N, Sendon-Lago J, Seoane S, Bermúdez MA, Lamelas ML, Garcia-Caballero T, et al. Potential therapeutic effect of the secretome from human uterine cervical stem cells against both cancer and stromal cells compared with adipose tissue stem cells. Oncotarget. 2014; 5:10692–708.
Article7. Yoon BS, Moon JH, Jun EK, Kim J, Maeng I, Kim JS, et al. Secretory profiles and wound healing effects of human amniotic fluid-derived mesenchymal stem cells. Stem Cells Dev. 2010; 19:887–902.
Article8. Shettles LB. Use of the Y chromosome in prenatal sex determination. Nature. 1971; 230:52–3.
Article9. Rizzo R, Vercammen M, van de Velde H, Horn PA, Rebmann V. The importance of HLA-G expression in embryos, trophoblast cells, and embryonic stem cells. Cell Mol Life Sci. 2011; 68:341–52.
Article10. Klingemann H, Matzilevich D, Marchand J. Mesenchymal stem cells - Sources and clinical applications. Transfus Med Hemother. 2008; 35:272–7.11. Fu WL, Zhou CY, Yu JK. A new source of mesenchymal stem cells for articular cartilage repair: MSCs derived from mobilized peripheral blood share similar biological characteristics in vitro and chondrogenesis in vivo as MSCs from bone marrow in a rabbit model. Am J Sports Med. 2014; 42:592–601.12. Han J, Koh YJ, Moon HR, Ryoo HG, Cho CH, Kim I, et al. Adipose tissue is an extramedullary reservoir for functional hematopoietic stem and progenitor cells. Blood. 2010; 115:957–64.
Article13. Huang YC, Yang ZM, Chen XH, Tan MY, Wang J, Li XQ, et al. Isolation of mesenchymal stem cells from human placental decidua basalis and resistance to hypoxia and serum deprivation. Stem Cell Rev Rep. 2009; 5:247–55.
Article14. Kassis I, Zangi L, Rivkin R, Levdansky L, Samuel S, Marx G, et al. Isolation of mesenchymal stem cells from G-CSFmobilized human peripheral blood using fibrin microbeads. Bone Marrow Transplant. 2006; 37:967–76.
Article15. Tsai MS, Lee JL, Chang YJ, Hwang SM. Isolation of human multipotent mesenchymal stem cells from secondtrimester amniotic fluid using a novel two-stage culture protocol. Hum Reprod. 2004; 19:1450–6.
Article16. Horwitz EM, Le Blanc K, Dominici M, Mueller I, SlaperCortenbach I, Marini FC, et al. Clarification of the nomenclature for MSC: the International Society for Cellular Therapy position statement. Cytotherapy. 2005; 7:393–5.
Article17. Wagner W, Wein F, Seckinger A, Frankhauser M, Wirkner U, Krause U, et al. Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Exp Hematol. 2005; 33:1402–16.
Article18. Lee RH, Kim B, Choi I, Kim H, Choi HS, Suh K, et al. Characterization and expression analysis of mesenchymal stem cells from human bone marrow and adipose tissue. Cell Physiol Biochem. 2004; 14:311–24.
Article19. Heo JS, Choi Y, Kim HS, Kim HO. Comparison of molecular profiles of human mesenchymal stem cells derived from bone marrow, umbilical cord blood, placenta and adipose tissue. Int J Mol Med. 2016; 37:115–25.
Article20. Riteau B, Rouas-Freiss N, Menier C, Paul P, Dausset J, Carosella ED. HLA-G2, -G3, and -G4 isoforms expressed as nonmature cell surface glycoproteins inhibit NK and antigen-specific CTL cytolysis. J Immunol. 2001; 166:5018–26.
Article21. Götherström C, West A, Liden J, Uzunel M, Lahesmaa R, Le Blanc K. Difference in gene expression between human fetal liver and adult bone marrow mesenchymal stem cells. Haematologica. 2005; 90:1017–26.22. Shi M, Liu ZW, Wang FS. Immunomodulatory properties and therapeutic application of mesenchymal stem cells. Clin Exp Immunol. 2011; 164:1–8.
Article23. Hviid TV. HLA-G in human reproduction: aspects of genetics, function and pregnancy complications. Hum Reprod Update. 2006; 12:209–32.
Article24. Yie SM, Taylor RN, Librach C. Low plasma HLA-G protein concentrations in early gestation indicate the development of preeclampsia later in pregnancy. Am J Obstet Gynecol. 2005; 193:204–8.
Article25. Goldman-Wohl DS, Ariel I, Greenfield C, Hochner-Celnikier D, Cross J, Fisher S, et al. Lack of human leukocyte antigen-G expression in extravillous trophoblasts is associated with pre-eclampsia. Mol Hum Reprod. 2000; 6:88–95.
Article26. Jain CV, Kadam L, van Dijk M, Kohan-Ghadr HR, Kilburn BA, Hartman C, et al. Fetal genome profiling at 5 weeks of gestation after noninvasive isolation of trophoblast cells from the endocervical canal. Sci Transl Med. 2016; 8:363. re4.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Concise Review: Differentiation of Human Adult Stem Cells Into Hepatocyte-like Cells In vitro
- Adipose Tissue Derived Mesenchymal Stem Cells
- A Study on the Relationship between Emotion Related to Pap Smear and Continuous Participation in Pap Smear Screening in Married Korean Women
- The comparison of Conventional Pap smears with Liquid Pap smears (PrepStain(TM) System) in Cervical Pap smears
- Adipose Tissue - Adequate, Accessible Regenerative Material