J Liver Cancer.
2020 Mar;20(1):84-89. 10.17998/jlc.20.1.84.
Successful Sequential Therapy Involving Regorafenib after Failure of Sorafenib in a Patient with Recurrent Hepatocellular Carcinoma after Liver Transplantation
- Affiliations
-
- 1Division of Hepatology, Department of Internal Medicine, Seoul St. Mary’s Hospital, Seoul, Korea
- 2Division of Hepatology, Department of Internal Medicine, Eunpyeong St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- KMID: 2505847
- DOI: http://doi.org/10.17998/jlc.20.1.84
Abstract
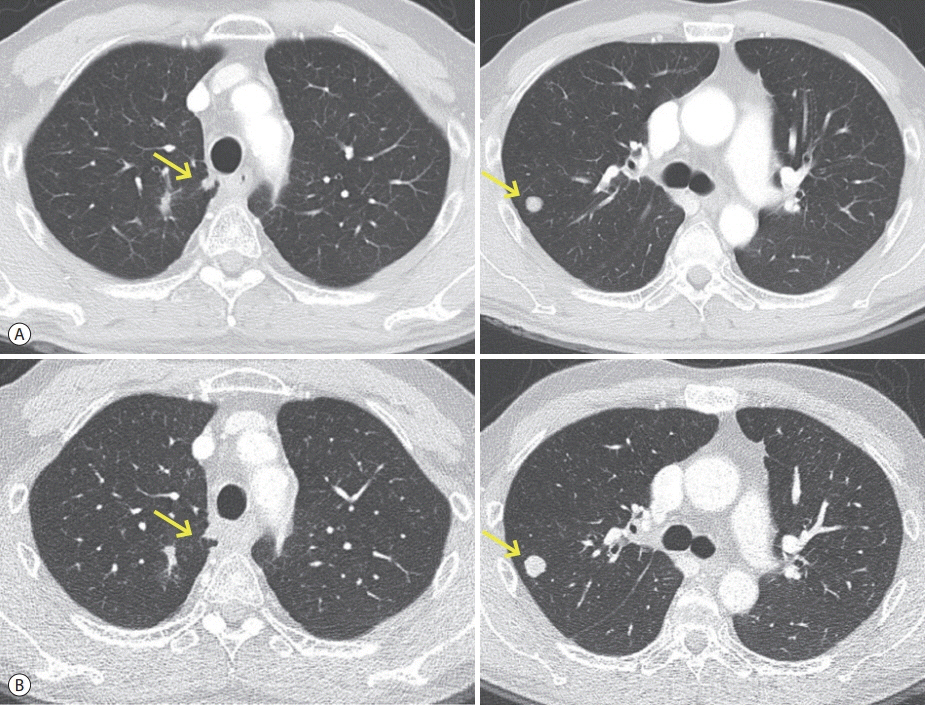
- The efficacy and safety of sequential systemic therapy for the treatment of recurrent hepatocellular carcinoma (HCC) after liver transplantation (LT) are not well established. This study describes a successful experience where sequential therapy with sorafenib followed by regorafenib was used to treat recurrent HCC in a 54-year old male LT recipient. After HCC recurred in both lungs 10 months after LT, sorafenib was administered with radiation therapy to treat pulmonary metastases. However, after 4 months of sorafenib treatment showed progressive pulmonary metastases, sequential regorafenib treatment was started. After 3 months (cycles) of regorafenib treatment, tumor response was partial, and after 6 months (cycles), disease status remained stable without signs of progression or drug-related serious adverse events. This case suggests that sequential systemic therapy is feasible in patient with recurrent HCC after LT.
Figure
Reference
-
1. Sapisochin G, Bruix J. Liver transplantation for hepatocellular carcinoma: outcomes and novel surgical approaches. Nat Rev Gastroenterol Hepatol. 2017; 14:203–217.2. Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996; 334:693–699.3. Yao FY, Xiao L, Bass NM, Kerlan R, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: validation of the UCSF-expanded criteria based on preoperative imaging. Am J Transplant. 2007; 7:2587–2596.4. Mazzaferro V, Llovet JM, Miceli R, Bhoori S, Schiavo M, Mariani L, et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009; 10:35–43.5. Kneuertz PJ, Cosgrove DP, Cameron AM, Kamel IR, Geschwind JF, Herman JM, et al. Multidisciplinary management of recurrent hepatocellular carcinoma following liver transplantation. J Gastrointest Surg. 2012; 16:874–881.6. European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of hepatocellular carcinoma. J Hepatol. 2018; 69:182–236.7. Korean Liver Cancer Association; National Cancer Center. 2018 Korean Liver Cancer Association-National Cancer Center Korea Practice Guidelines for the Management of Hepatocellular Carcinoma. Gut Liver. 2019; 13:227–299.8. Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018; 67:358–380.9. Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982; 5:649–655.10. Wilhelm SM, Dumas J, Adnane L, Lynch M, Carter CA, Schutz G, et al. Regorafenib (BAY 73-4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer. 2011; 129:245–255.11. Abou-Elkacem L, Arns S, Brix G, Gremse F, Zopf D, Kiessling F, et al. Regorafenib inhibits growth, angiogenesis, and metastasis in a highly aggressive, orthotopic colon cancer model. Mol Cancer Ther. 2013; 12:1322–1331.12. Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004; 64:7099–7109.13. Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, doubleblind, placebo-controlled, phase 3 trial. Lancet. 2017; 389:56–66.14. Finn RS, Merle P, Granito A, Huang YH, Bodoky G, Pracht M, et al. Outcomes of sequential treatment with sorafenib followed by regorafenib for HCC: additional analyses from the phase III RESORCE trial. J Hepatol. 2018; 69:353–358.15. Iavarone M, Invernizzi F, Czauderna C, Sanduzzi-Zamparelli M, Bhoori S, Amaddeo G, et al. Preliminary experience on safety of regorafenib after sorafenib failure in recurrent hepatocellular carcinoma after liver transplantation. Am J Transplant. 2019; 19:3176–3184.16. Rimassa L, Pressiani T, Merle P. Systemic treatment options in hepatocellular carcinoma. Liver Cancer. 2019; 8:427–446.17. Vogel A, Saborowski A. Current strategies for the treatment of intermediate and advanced hepatocellular carcinoma. Cancer Treat Rev. 2019; 82:101946.18. Halazun KJ, Najjar M, Abdelmessih RM, Samstein B, Griesemer AD, Guarrera JV, et al. Recurrence after liver transplantation for hepatocellular carcinoma: a new MORAL to the story. Ann Surg. 2017; 265:557–564.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Sequential regorafenib or nivolumab therapy in recurrent hepatocellular carcinoma with sorafenib failure in liver transplant patients does not improve prognosis
- Systemic Therapy for Advanced Hepatocellular Carcinoma: Targeted Therapy and Immunotherapy
- An Analysis for Survival Predictors for Patients with Hepatocellular Carcinoma Who Failed to Sorafenib Treatment in Pre-regorafenib Era
- Nivolumab for Advanced Hepatocellular Carcinoma with Multiple Lung Metastases after Sorafenib Failure
- Sorafenib for Recurrent Hepatocellular Carcinoma after Liver Transplantation