Korean J Physiol Pharmacol.
2020 Sep;24(5):433-440. 10.4196/kjpp.2020.24.5.433.
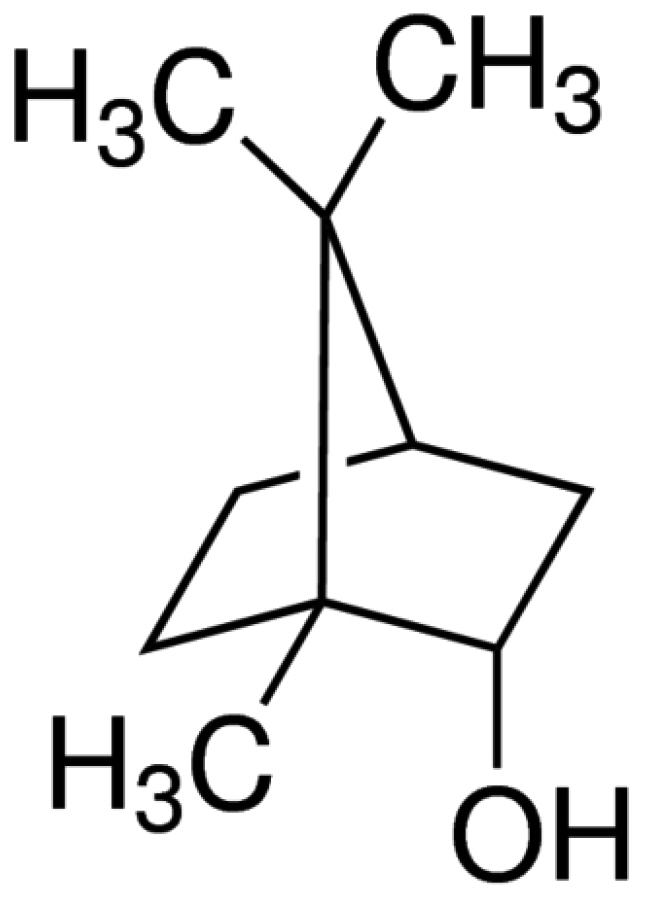
Inhibitory actions of borneol on the substantia gelatinosa neurons of the trigeminal subnucleus caudalis in mice
- Affiliations
-
- 1Department of Oral Physiology, School of Dentistry & Institute of Oral Bioscience, Jeonbuk National University, Jeonju 54896, Korea
- 2Faculty of Odonto- Stomatology, Hue University of Medicine and Pharmacy, Hue University, Hue 53000, Vietnam
- KMID: 2505778
- DOI: http://doi.org/10.4196/kjpp.2020.24.5.433
Abstract
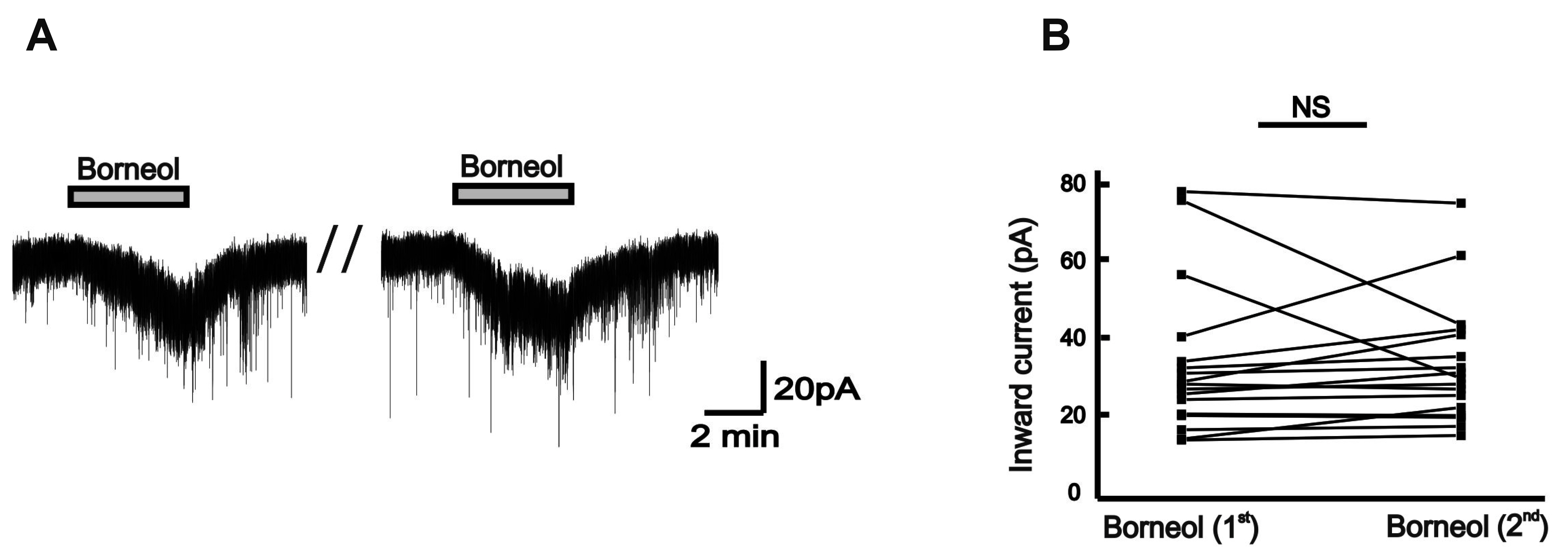
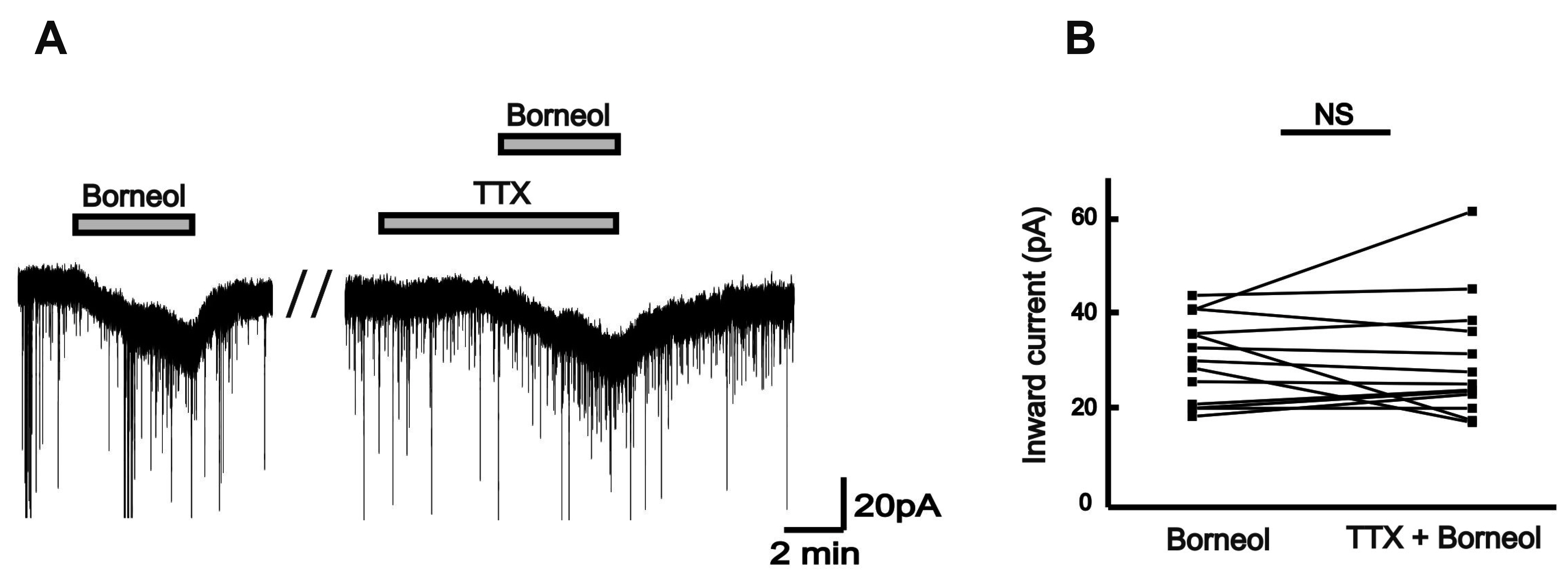
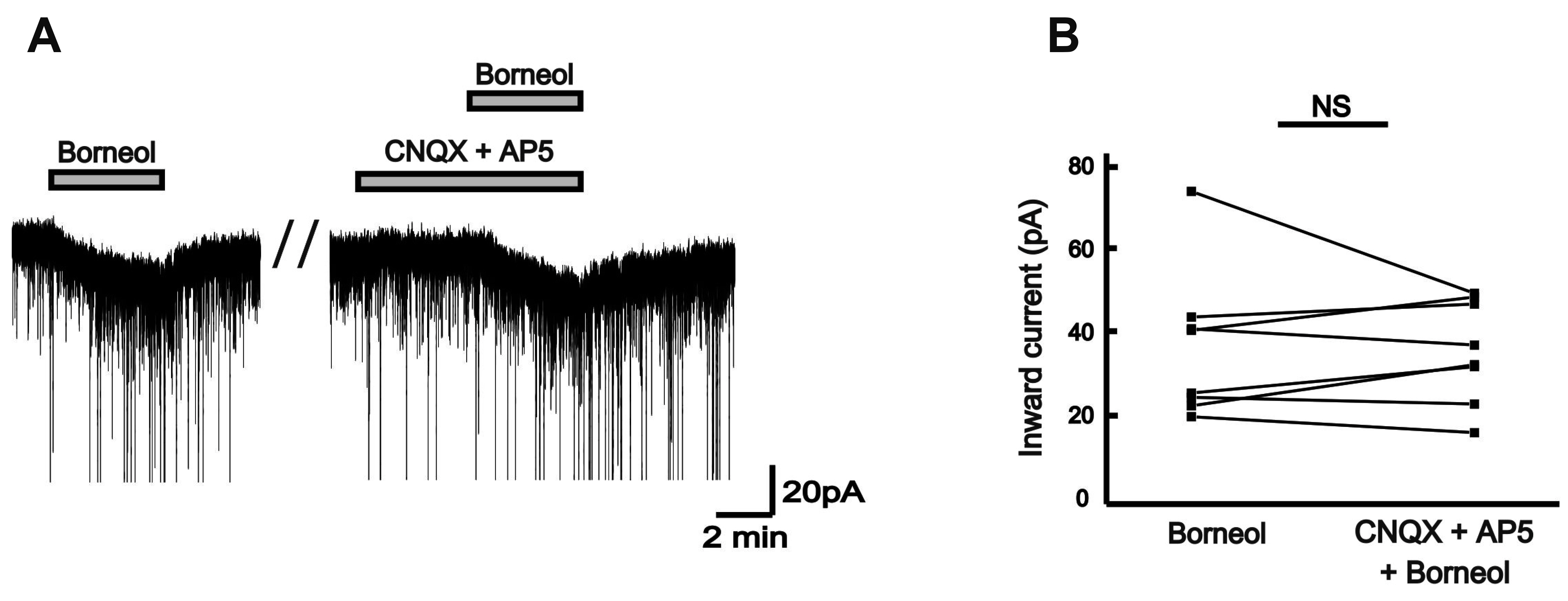
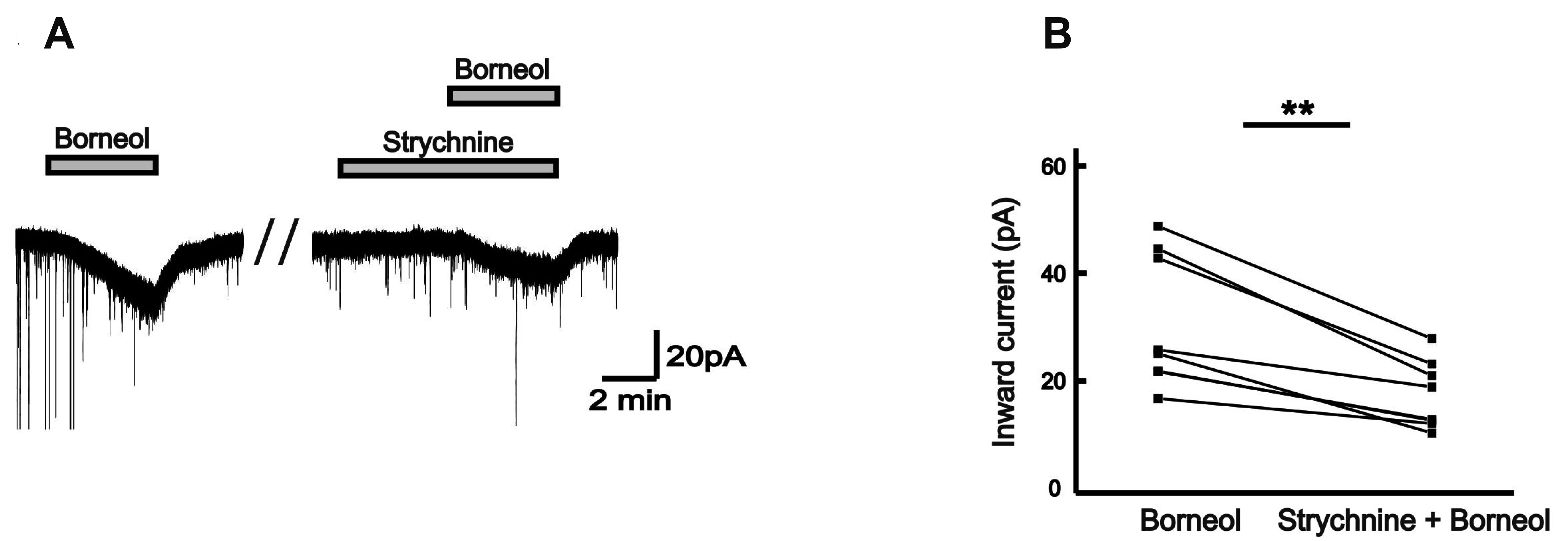
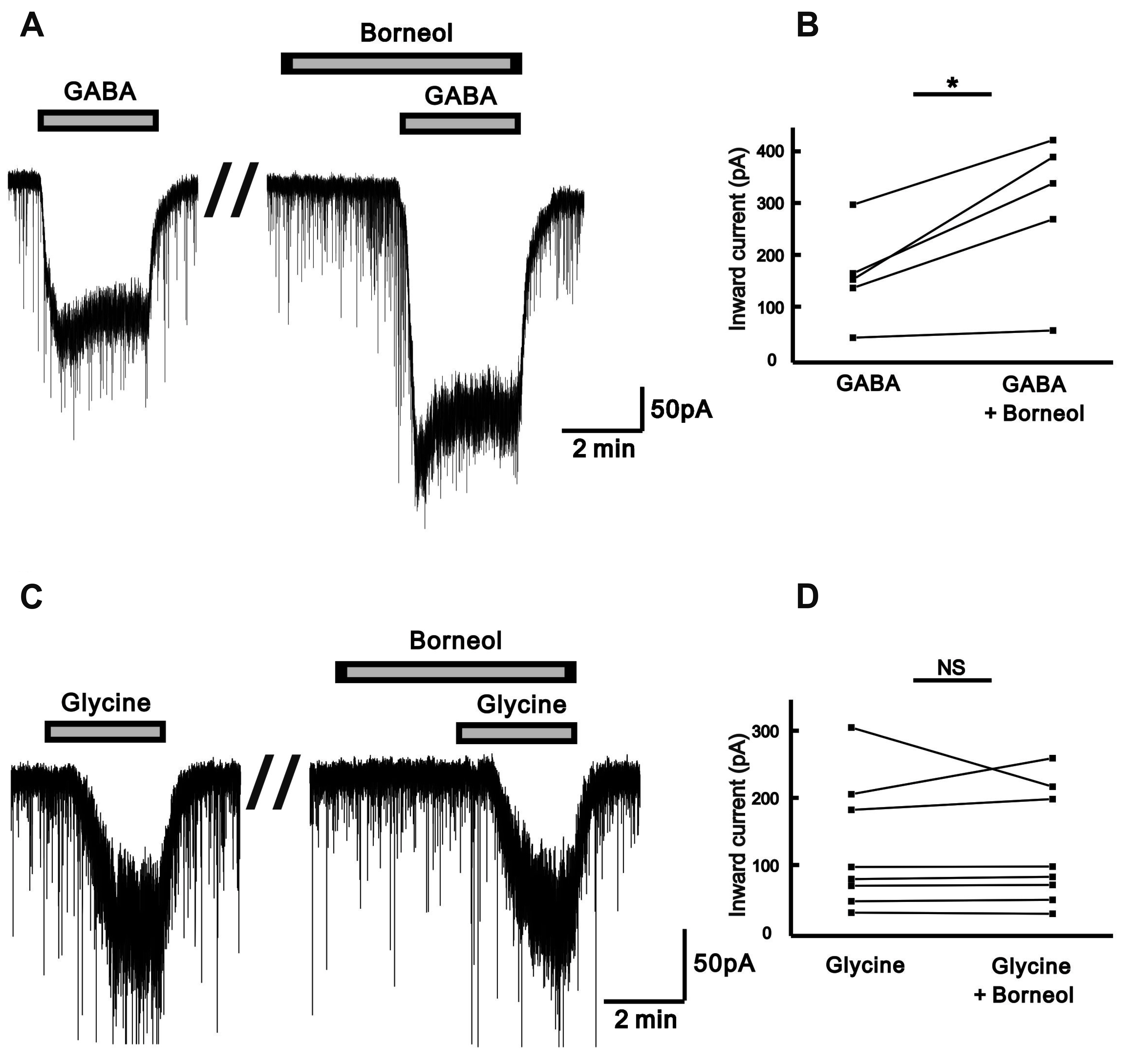
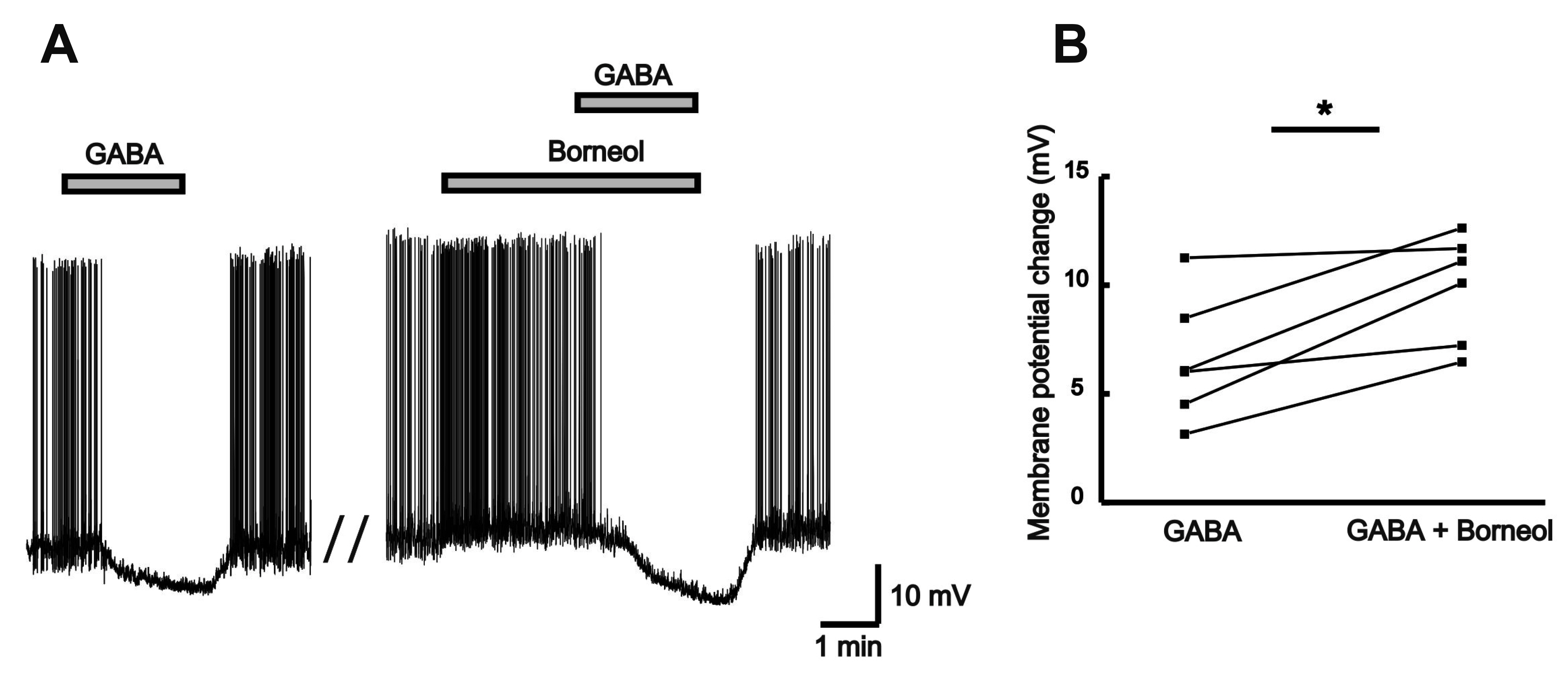
- The substantia gelatinosa (SG) of the trigeminal subnucleus caudalis(Vc) is the first relay site for the orofacial nociceptive inputs via the thin myelinatedAδ and unmyelinated C primary afferent fibers. Borneol, one of the valuable timehonoredherbal ingredients in traditional Chinese medicine, is a popular treatmentfor anxiety, anesthesia, and antinociception. However, to date, little is known asto how borneol acts on the SG neurons of the Vc. To close this gap, the whole-cellpatch-clamp technique was applied to elucidate the antinociceptive mechanismresponding for the actions of borneol on the SG neurons of the Vc in mice. In thevoltage-clamp mode, holding at –60 mV, the borneol-induced non-desensitizinginward currents were not affected by tetrodotoxin, a voltage-gated Na+ channelblocker, 6-cyano-7-nitro-quinoxaline-2,3-dione, a non-N-methyl-D-aspartate (NMDA)glutamate receptor antagonist and DL-2-amino-5-phosphonopentanoic acid, anNMDA receptor antagonist. However, borneol-induced inward currents were partiallydecreased in the presence of picrotoxin, a -aminobutyric acid (GABA)A receptorantagonist, or strychnine, a glycine receptor antagonist, and was almost suppressedin the presence of picrotoxin and strychnine. Though borneol did not show any effecton the glycine-induced inward currents, borneol enhanced GABA-mediatedresponses. Beside, borneol enhanced the GABA-induced hyperpolarization under thecurrent-clamp mode. Altogether, we suggest that borneol contributes in part towardmediating the inhibitory GABA and glycine transmission on the SG neurons of the Vcand may serve as an herbal therapeutic for orofacial pain ailments.
Figure
Reference
-
1. Romero-Reyes M, Uyanik JM. 2014; Orofacial pain management: current perspectives. J Pain Res. 7:99–115. DOI: 10.2147/JPR.S37593. PMID: 24591846. PMCID: PMC3937250.
Article2. Melzack R, Wall PD. 1965; Pain mechanisms: a new theory. Science. 150(3699):971–979. DOI: 10.1126/science.150.3699.971. PMID: 5320816.
Article3. Tsai CM, Chiang CY, Yu XM, Sessle BJ. 1999; Involvement of trigeminal subnucleus caudalis (medullary dorsal horn) in craniofacial nociceptive reflex activity. Pain. 81:115–128. DOI: 10.1016/S0304-3959(99)00009-3. PMID: 10353499.
Article4. Ren K, Dubner R. 2011; The role of trigeminal interpolaris-caudalis transition zone in persistent orofacial pain. Int Rev Neurobiol. 97:207–225. DOI: 10.1016/B978-0-12-385198-7.00008-4. PMID: 21708312. PMCID: PMC3257052.
Article5. Sessle BJ. 2000; Acute and chronic craniofacial pain: brainstem mechanisms of nociceptive transmission and neuroplasticity, and their clinical correlates. Crit Rev Oral Biol Med. 11:57–91. DOI: 10.1177/10454411000110010401. PMID: 10682901.
Article6. Hattori A. 2000; [Camphor in the Edo era - camphor and borneol for medicines]. Yakushigaku Zasshi. 35:49–54. Japanese.7. Almeida JR, Souza GR, Silva JC, Saraiva SR, Júnior RG, Quintans Jde S, Barreto Rde S, Bonjardim LR, Cavalcanti SC, Quintans LJ Jr. 2013; Borneol, a bicyclic monoterpene alcohol, reduces nociceptive behavior and inflammatory response in mice. ScientificWorldJournal. 2013:808460. DOI: 10.1155/2013/808460. PMID: 23710149. PMCID: PMC3654274.
Article8. Bhatia SP, Letizia CS, Api AM. 2008; Fragrance material review on borneol. Food Chem Toxicol. 46(Suppl 11):S77–S80. DOI: 10.1016/j.fct.2008.06.031. PMID: 18640181.
Article9. Jiang J, Shen YY, Li J, Lin YH, Luo CX, Zhu DY. 2015; (+)-Borneol alleviates mechanical hyperalgesia in models of chronic inflammatory and neuropathic pain in mice. Eur J Pharmacol. 757:53–58. DOI: 10.1016/j.ejphar.2015.03.056. PMID: 25835611.
Article10. Zhou HH, Zhang L, Zhou QG, Fang Y, Ge WH. 2016; (+)-Borneol attenuates oxaliplatin-induced neuropathic hyperalgesia in mice. Neuroreport. 27:160–165. DOI: 10.1097/WNR.0000000000000516. PMID: 26730517.
Article11. Nesterkina M, Kravchenko I. 2017; Synthesis and pharmacological properties of novel esters based on monoterpenoids and glycine. Pharmaceuticals (Basel). 10:47. DOI: 10.3390/ph10020047. PMID: 28524111. PMCID: PMC5490404.
Article12. Takaishi M, Uchida K, Fujita F, Tominaga M. 2014; Inhibitory effects of monoterpenes on human TRPA1 and the structural basis of their activity. J Physiol Sci. 64:47–57. DOI: 10.1007/s12576-013-0289-0. PMID: 24122170. PMCID: PMC3889502.
Article13. Nguyen TTP, Jang SH, Park SJ, Cho DH, Han SK. 2019; Action of citral on the substantia gelatinosa neurons of the trigeminal subnucleus caudalis in juvenile mice. Chin J Physiol. 62:175–181. DOI: 10.4103/CJP.CJP_32_19. PMID: 31670280.
Article14. Katz B, Miledi R. 1969; Tetrodotoxin-resistant electric activity in presynaptic terminals. J Physiol. 203:459–487. DOI: 10.1113/jphysiol.1969.sp008875. PMID: 4307710. PMCID: PMC1351456.
Article15. Narahashi T. 1974; Chemicals as tools in the study of excitable membranes. Physiol Rev. 54:813–889. DOI: 10.1152/physrev.1974.54.4.813. PMID: 4153804.
Article16. Carpenter TS, Lau EY, Lightstone FC. 2013; Identification of a possible secondary picrotoxin-binding site on the GABAA receptor. Chem Res Toxicol. 26:1444–1454. DOI: 10.1021/tx400167b. PMID: 24028067.17. Dutertre S, Becker CM, Betz H. 2012; Inhibitory glycine receptors: an update. J Biol Chem. 287:40216–40223. DOI: 10.1074/jbc.R112.408229. PMID: 23038260. PMCID: PMC3504737.
Article18. Todd AJ, Watt C, Spike RC, Sieghart W. 1996; Colocalization of GABA, glycine, and their receptors at synapses in the rat spinal cord. J Neurosci. 16:974–982. DOI: 10.1523/JNEUROSCI.16-03-00974.1996. PMID: 8558266. PMCID: PMC6578783.
Article19. Granger RE, Campbell EL, Johnston GA. 2005; (+)- and (-)-borneol: efficacious positive modulators of GABA action at human recombinant alpha1beta2gamma2L GABAA receptors. Biochem Pharmacol. 69:1101–1111. DOI: 10.1016/j.bcp.2005.01.002. PMID: 15763546.20. Sherkheli MA, Schreiner B, Haq R, Werner M, Hatt H. 2015; Borneol inhibits TRPA1, a proinflammatory and noxious pain-sensing cation channel. Pak J Pharm Sci. 28:1357–1363. PMID: 26142526.21. Chen GL, Lei M, Zhou LP, Zeng B, Zou F. 2016; Borneol is a TRPM8 agonist that increases ocular surface wetness. PLoS One. 11:e0158868. DOI: 10.1371/journal.pone.0158868. PMID: 27448228. PMCID: PMC4957794.
Article22. Chinese Pharmacopoeia Commission. 2010. Pharmacopoeia of the People's Republic of China. China Medical Science Press;Beijing: p. 161–162.23. Ji J, Zhang R, Li H, Zhu J, Pan Y, Guo Q. 2020; Analgesic and anti-inflammatory effects and mechanism of action of borneol on photodynamic therapy of acne. Environ Toxicol Pharmacol. 75:103329. DOI: 10.1016/j.etap.2020.103329. PMID: 31978868.
Article24. Bowery NG, Smart TG. 2006; GABA and glycine as neurotransmitters: a brief history. Br J Pharmacol. 147(Suppl 1):S109–S119. DOI: 10.1038/sj.bjp.0706443. PMID: 16402094. PMCID: PMC1760744.
Article25. Cao B, Ni HY, Li J, Zhou Y, Bian XL, Tao Y, Cai CY, Qin C, Wu HY, Chang L, Luo CX, Zhu DY. 2018; (+)-Borneol suppresses conditioned fear recall and anxiety-like behaviors in mice. Biochem Biophys Res Commun. 495:1588–1593. DOI: 10.1016/j.bbrc.2017.12.025. PMID: 29223397.
Article26. Zhang N, Liu P, He X. 2011; [Effect of single-used borneol and combining it with diazepam on content of neurotransmitter in corpus striatum of rats]. Zhongguo Zhong Yao Za Zhi. 36:3180–3183. Chinese. PMID: 22375403.27. Hall AC, Turcotte CM, Betts BA, Yeung WY, Agyeman AS, Burk LA. 2004; Modulation of human GABAA and glycine receptor currents by menthol and related monoterpenoids. Eur J Pharmacol. 506:9–16. DOI: 10.1016/j.ejphar.2004.10.026. PMID: 15588619.28. Todd AJ, Sullivan AC. 1990; Light microscope study of the coexistence of GABA-like and glycine-like immunoreactivities in the spinal cord of the rat. J Comp Neurol. 296:496–505. DOI: 10.1002/cne.902960312. PMID: 2358549.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression of KA1 kainate receptor subunit in the substantia gelatinosa of the trigeminal subnucleus caudalis in mice
- Potentiation of the glycine response by serotonin on the substantia gelatinosa neurons of the trigeminal subnucleus caudalis in mice
- Naringenin modulates GABA mediated response in a sexdependent manner in substantia gelatinosa neurons of trigeminal subnucleus caudalis in immature mice
- Glycine- and GABA-mimetic Actions of Shilajit on the Substantia Gelatinosa Neurons of the Trigeminal Subnucleus Caudalis in Mice
- Botulinum toxin type A enhances the inhibitory spontaneous postsynaptic currents on the substantia gelatinosa neurons of the subnucleus caudalis in immature mice