Korean J Physiol Pharmacol.
2020 Sep;24(5):403-412. 10.4196/kjpp.2020.24.5.403.
Cilostazol ameliorates diabetic nephropathy by inhibiting highglucose- induced apoptosis
- Affiliations
-
- 1Division of Nephrology, Department of Paediatrics, Changhua Christian Hospital, Changhua 500, Taiwan
- 2Department of Urology, Taipei City Hospital, Taipei 10341, Taiwan
- 3Department of Pathology, Chung Shan Medical University Hospital, Taichung 40221, Taiwan
- 4Department of Medical Research, Changhua Christian Hospital, Changhua 500, Taiwan
- 5Department of Clinical Biochemistry, Chung Shan Medical University Hospital, Taichung 40221, Taiwan
- 6Division of Nephropathy, Department of Internal Medicine, Chang Bing Show-Chwan Memborial Hospital, Changhua 505, Taiwan
- 7Institute of Biochemistry, Microbiology and Immunology, Medical College, Chung Shan Medical University, Taichung 40221, Taiwan
- 8Department of Biochemistry, School of Medicine, College of Medicine, Chung Shan Medical University, Taichung 40221, Taiwan
- KMID: 2505775
- DOI: http://doi.org/10.4196/kjpp.2020.24.5.403
Abstract
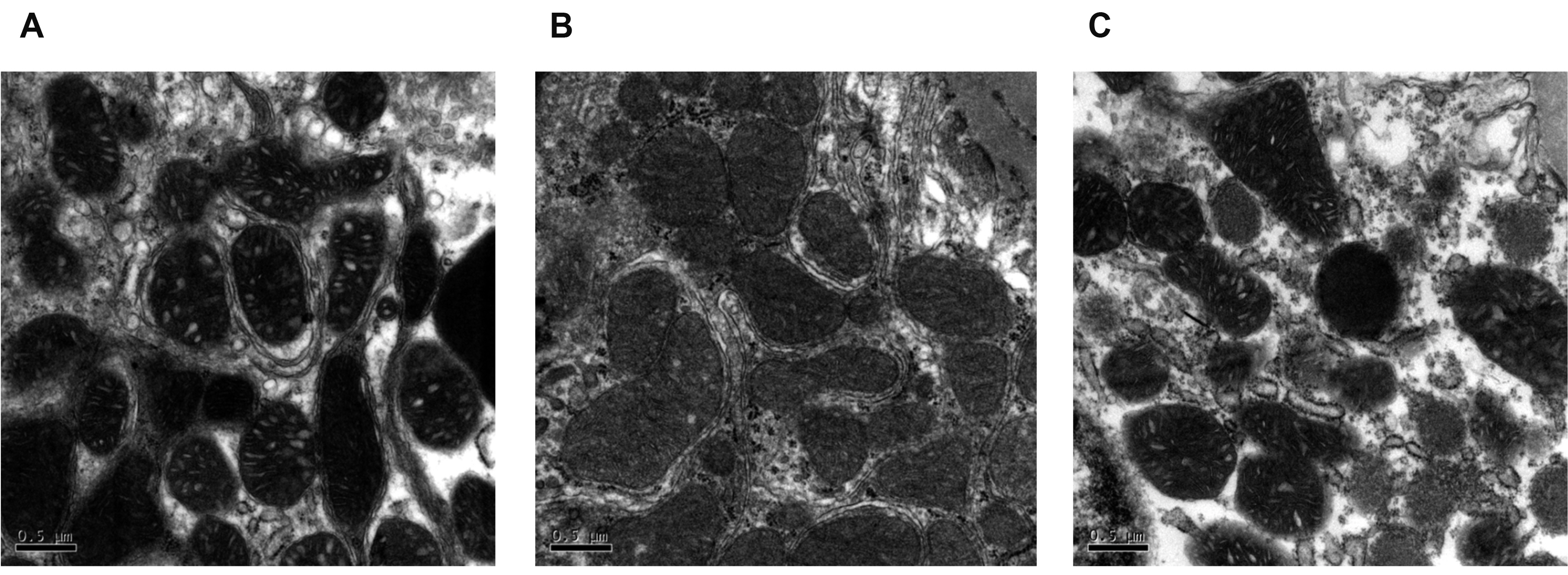
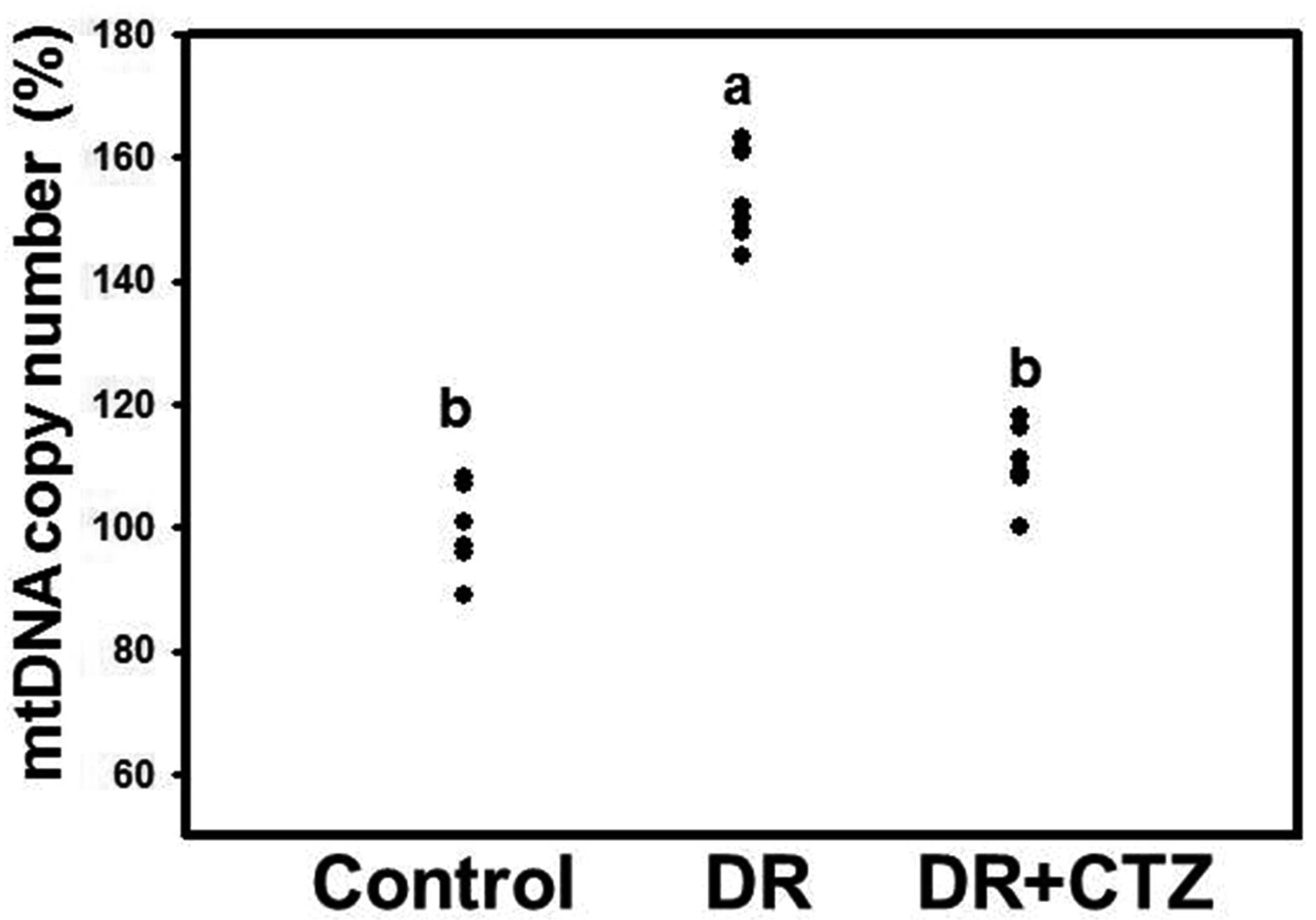
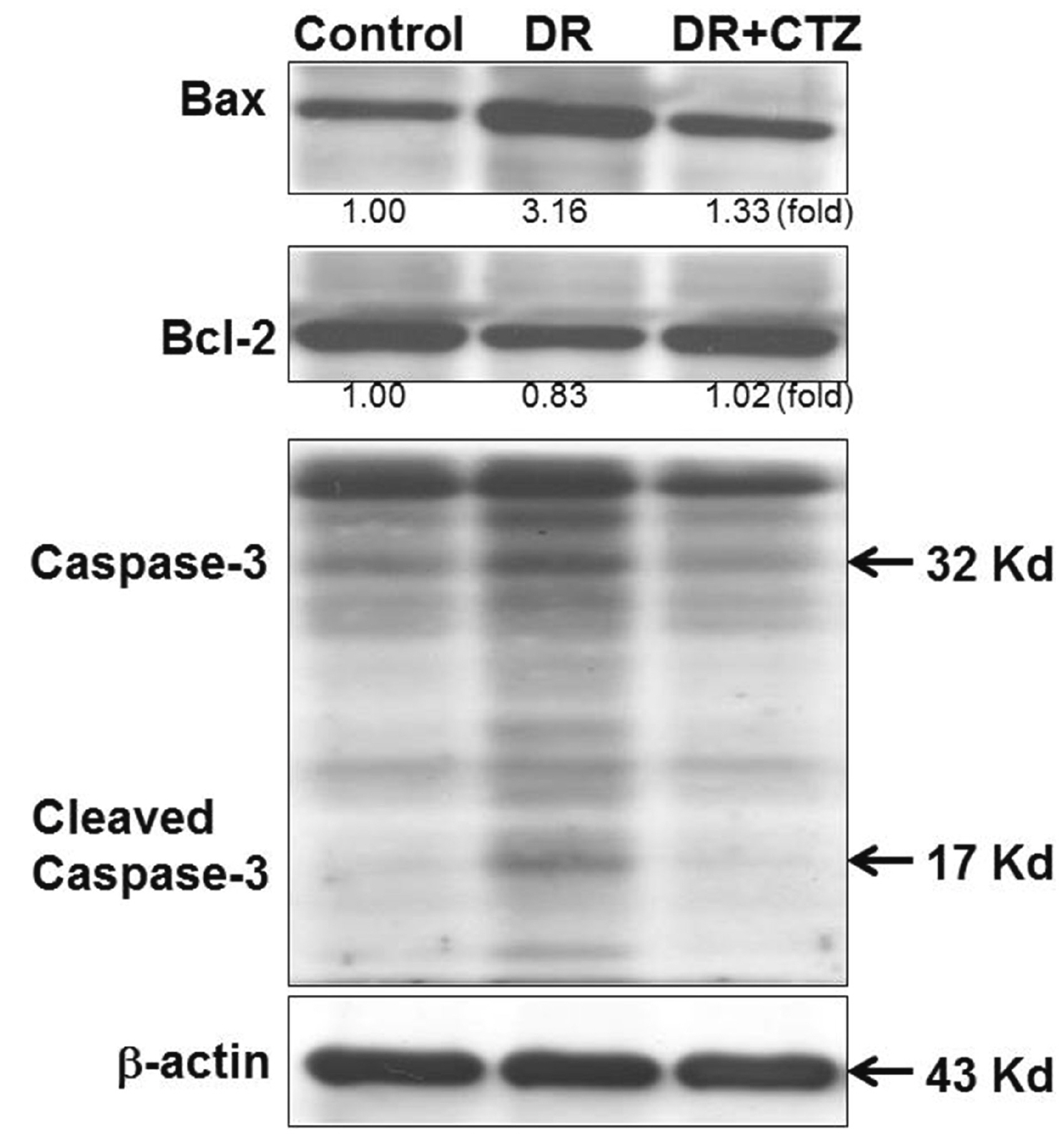
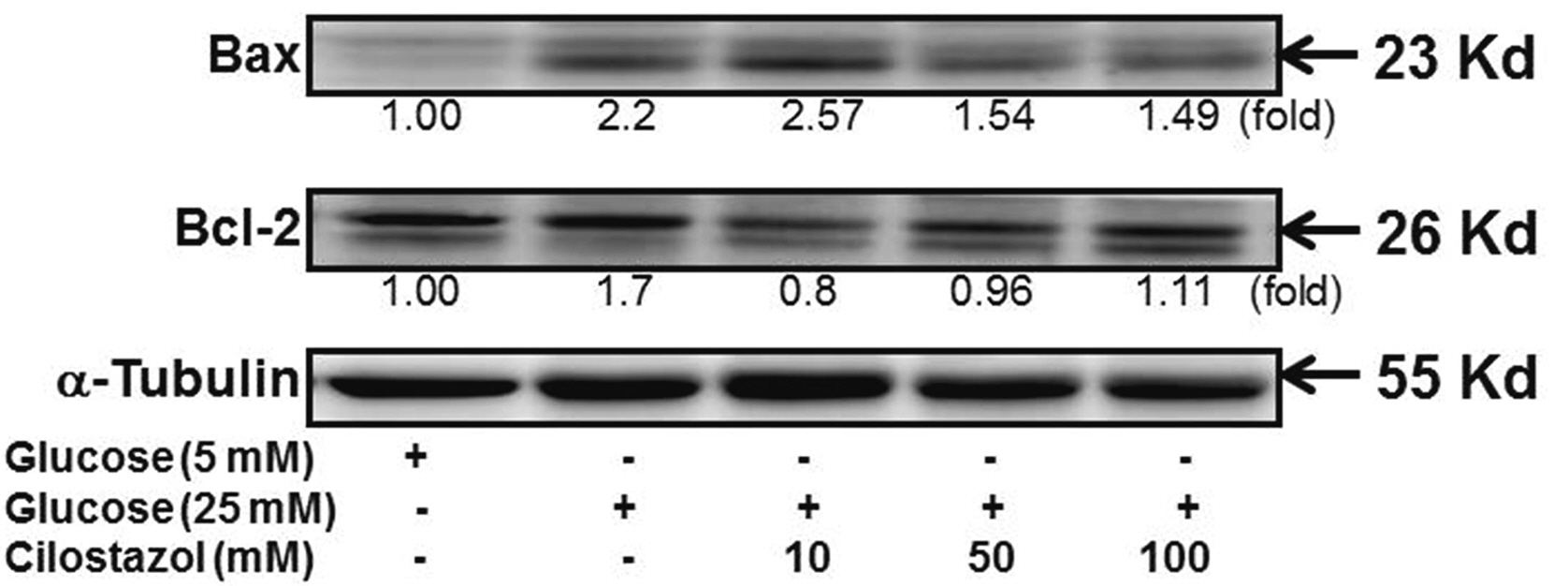
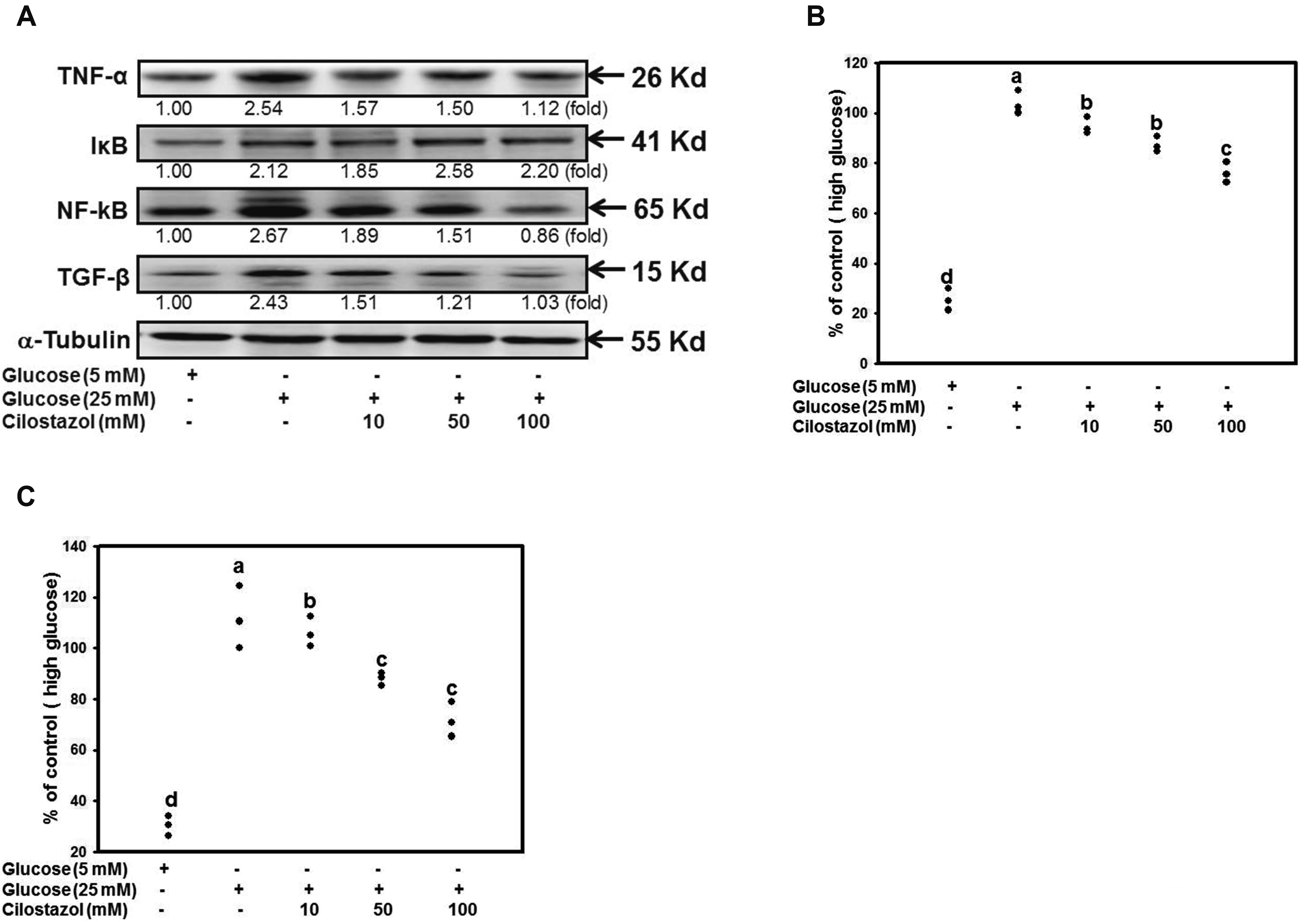
- Diabetic nephropathy (DN) is a hyperglycemia-induced progressivedevelopment of renal insufficiency. Excessive glucose can increase mitochondrialreactive oxygen species (ROS) and induce cell damage, causing mitochondrial dysfunction.Our previous study indicated that cilostazol (CTZ) can reduce ROS levelsand decelerate DN progression in streptozotocin (STZ)-induced type 1 diabetes.This study investigated the potential mechanisms of CTZ in rats with DN and in highglucose-treated mesangial cells. Male Sprague–Dawley rats were fed 5 mg/kg/day ofCTZ after developing STZ-induced diabetes mellitus. Electron microscopy revealedthat CTZ reduced the thickness of the glomerular basement membrane and improvedmitochondrial morphology in mesangial cells of diabetic kidney. CTZ treatmentreduced excessive kidney mitochondrial DNA copy numbers induced by hyperglycemiaand interacted with the intrinsic pathway for regulating cell apoptosis as anantiapoptotic mechanism. In high-glucose-treated mesangial cells, CTZ reduced ROSproduction, altered the apoptotic status, and down-regulated transforming growthfactor beta (TGF-) and nuclear factor kappa light chain enhancer of activated B cells(NF-B). Base on the results of our previous and current studies, CTZ decelerationof hyperglycemia-induced DN is attributable to ROS reduction and thereby maintenanceof the mitochondrial function and reduction in TGF- and NF-B levels.
Figure
Reference
-
1. Grubb E, Van der Vaart J, Norris K, Smoyer KE, Rolland C. 2016; Albuminuria and serum creatinine in predicting renal function decline in patients with diabetic nephropathy: a systematic literature review. Value Health. 19:PA520. DOI: 10.1016/j.jval.2016.09.1007.
Article2. Papadopoulou-Marketou N, Chrousos GP, Kanaka-Gantenbein C. 2017; Diabetic nephropathy in type 1 diabetes: a review of early natural history, pathogenesis, and diagnosis. Diabetes Metab Res Rev. 33:e2841. DOI: 10.1002/dmrr.2841. PMID: 27457509.
Article3. Kitada M, Ogura Y, Suzuki T, Sen S, Lee SM, Kanasaki K, Kume S, Koya D. 2016; A very-low-protein diet ameliorates advanced diabetic nephropathy through autophagy induction by suppression of the mTORC1 pathway in Wistar fatty rats, an animal model of type 2 diabetes and obesity. Diabetologia. 59:1307–1317. DOI: 10.1007/s00125-016-3925-4. PMID: 27020449.
Article4. Marshall CB. 2016; Rethinking glomerular basement membrane thickening in diabetic nephropathy: adaptive or pathogenic? Am J Physiol Renal Physiol. 311:F831–F843. DOI: 10.1152/ajprenal.00313.2016. PMID: 27582102. PMCID: PMC6121820.
Article5. Wang Z, do Carmo JM, Hall JE. 2016; ER stress and mitochondrial ROS contribute to the development of hypertensive‐diabetic nephropathy. FASEB J. 30(Suppl 1):740.17.6. Vokurková M, Rauchová H, Řezáčová L, Vaněčková I, Zicha J. 2015; NADPH oxidase activity and reactive oxygen species production in brain and kidney of adult male hypertensive Ren-2 transgenic rats. Physiol Res. 64:849–856. DOI: 10.33549/physiolres.933254. PMID: 26713567.
Article7. Jha JC, Thallas-Bonke V, Banal C, Gray SP, Chow BS, Ramm G, Quaggin SE, Cooper ME, Schmidt HH, Jandeleit-Dahm KA. 2016; Podocyte-specific Nox4 deletion affords renoprotection in a mouse model of diabetic nephropathy. Diabetologia. 59:379–389. DOI: 10.1007/s00125-015-3796-0. PMID: 26508318. PMCID: PMC6450410.
Article8. Gnudi L, Coward RJM, Long DA. 2016; Diabetic nephropathy: perspective on novel molecular mechanisms. Trends Endocrinol Metab. 27:820–830. DOI: 10.1016/j.tem.2016.07.002. PMID: 27470431.
Article9. Lee WC, Chen HC, Wang CY, Lin PY, Ou TT, Chen CC, Wen MC, Wang J, Lee HJ. 2010; Cilostazol ameliorates nephropathy in type 1 diabetic rats involving improvement in oxidative stress and regulation of TGF-Beta and NF-kappaB. Biosci Biotechnol Biochem. 74:1355–1361. DOI: 10.1271/bbb.90938. PMID: 20622454.10. Zhai YP, Lu Q, Liu YW, Cheng Q, Wei YQ, Zhang F, Li CL, Yin XX. 2013; Over-production of nitric oxide by oxidative stress-induced activation of the TGF-β1/PI3K/Akt pathway in mesangial cells cultured in high glucose. Acta Pharmacol Sin. 34:507–514. DOI: 10.1038/aps.2012.207. PMID: 23524565. PMCID: PMC4002794.
Article11. Nishikawa T, Brownlee M, Araki E. 2015; Mitochondrial reactive oxygen species in the pathogenesis of early diabetic nephropathy. J Diabetes Investig. 6:137–139. DOI: 10.1111/jdi.12258. PMID: 25802720. PMCID: PMC4364847.
Article12. Mengel-From J, Thinggaard M, Dalgård C, Kyvik KO, Christensen K, Christiansen L. 2014; Mitochondrial DNA copy number in peripheral blood cells declines with age and is associated with general health among elderly. Hum Genet. 133:1149–1159. DOI: 10.1007/s00439-014-1458-9. PMID: 24902542. PMCID: PMC4127366.
Article13. Shan Z, Chen S, Sun T, Luo C, Guo Y, Yu X, Yang W, Hu FB, Liu L. 2016; U-shaped association between plasma manganese levels and type 2 diabetes. Environ Health Perspect. 124:1876–1881. DOI: 10.1289/EHP176. PMID: 27258818. PMCID: PMC5132633.
Article14. Lee HC, Wei YH. 2005; Mitochondrial biogenesis and mitochondrial DNA maintenance of mammalian cells under oxidative stress. Int J Biochem Cell Biol. 37:822–834. DOI: 10.1016/j.biocel.2004.09.010. PMID: 15694841.
Article15. Yoon CY, Park JT, Kee YK, Han SG, Han IM, Kwon YE, Park KS, Lee MJ, Han SH, Kang SW, Yoo TH. 2016; Low mitochondrial DNA copy number is associated with adverse clinical outcomes in peritoneal dialysis patients. Medicine (Baltimore). 95:e2717. DOI: 10.1097/MD.0000000000002717. PMID: 26886611. PMCID: PMC4998611.
Article16. Al-Kafaji G, Golbahar J. 2013; High glucose-induced oxidative stress increases the copy number of mitochondrial DNA in human mesangial cells. Biomed Res Int. 2013:754946. DOI: 10.1155/2013/754946. PMID: 23984405. PMCID: PMC3745925.
Article17. Jeon YH, Heo YS, Kim CM, Hyun YL, Lee TG, Ro S, Cho JM. 2005; Phosphodiesterase: overview of protein structures, potential therapeutic applications and recent progress in drug development. Cell Mol Life Sci. 62:1198–1220. DOI: 10.1007/s00018-005-4533-5. PMID: 15798894.
Article18. Soderling SH, Beavo JA. 2000; Regulation of cAMP and cGMP signaling: new phosphodiesterases and new functions. Curr Opin Cell Biol. 12:174–179. DOI: 10.1016/S0955-0674(99)00073-3. PMID: 10712916.
Article19. Cheng J, Grande JP. 2007; Cyclic nucleotide phosphodiesterase (PDE) inhibitors: novel therapeutic agents for progressive renal disease. Exp Biol Med (Maywood). 232:38–51. PMID: 17202584.20. Cheng J, Thompson MA, Walker HJ, Gray CE, Diaz Encarnacion MM, Warner GM, Grande JP. 2004; Differential regulation of mesangial cell mitogenesis by cAMP phosphodiesterase isozymes 3 and 4. Am J Physiol Renal Physiol. 287:F940–F953. DOI: 10.1152/ajprenal.00079.2004. PMID: 15280158.
Article21. Matsumoto T, Kobayashi T, Wakabayashi K, Kamata K. 2005; Cilostazol improves endothelium-derived hyperpolarizing factor-type relaxation in mesenteric arteries from diabetic rats. Am J Physiol Heart Circ Physiol. 289:H1933–H1940. DOI: 10.1152/ajpheart.00303.2005. PMID: 15908466.
Article22. Tsai YC, Kuo PL, Hung WW, Wu LY, Wu PH, Chang WA, Kuo MC, Hsu YL. 2018; Angpt2 induces mesangial cell apoptosis through the microRNA-33-5p-SOCS5 loop in diabetic nephropathy. Mol Ther Nucleic Acids. 13:543–555. DOI: 10.1016/j.omtn.2018.10.003. PMID: 30414568. PMCID: PMC6226567.
Article23. Chae YM, Park KK, Magae J, Lee IS, Kim CH, Kim HC, Hong S, Lee JG, Choi IJ, Kim HS, Min KS, Lee IK, Chang YC. 2004; Sp1-decoy oligodeoxynucleotide inhibits high glucose-induced mesangial cell proliferation. Biochem Biophys Res Commun. 319:550–555. DOI: 10.1016/j.bbrc.2004.05.025. PMID: 15178441.
Article24. Wolf G, Ziyadeh FN. 1999; Molecular mechanisms of diabetic renal hypertrophy. Kidney Int. 56:393–405. DOI: 10.1046/j.1523-1755.1999.00590.x. PMID: 10432377.
Article25. Young BA, Johnson RJ, Alpers CE, Eng E, Gordon K, Floege J, Couser WG, Seidel K. 1995; Cellular events in the evolution of experimental diabetic nephropathy. Kidney Int. 47:935–944. DOI: 10.1038/ki.1995.139. PMID: 7752595.
Article26. Rahman MH, Jha MK, Suk K. 2016; Evolving insights into the pathophysiology of diabetic neuropathy: implications of malfunctioning glia and discovery of novel therapeutic targets. Curr Pharm Des. 22:738–757. DOI: 10.2174/1381612822666151204001234. PMID: 26635266.27. Zhao JH. 2019; Mesangial cells and renal fibrosis. Adv Exp Med Biol. 1165:165–194. DOI: 10.1007/978-981-13-8871-2_9. PMID: 31399966.
Article28. Sun Z, Ma Y, Chen F, Wang S, Chen B, Shi J. 2018; Artesunate ameliorates high glucose-induced rat glomerular mesangial cell injury by suppressing the TLR4/NF-κB/NLRP3 inflammasome pathway. Chem Biol Interact. 293:11–19. DOI: 10.1016/j.cbi.2018.07.011. PMID: 30031708.
Article29. Yao L, Li J, Li L, Li X, Zhang R, Zhang Y, Mao X. 2019; Coreopsis tinctoria Nutt ameliorates high glucose-induced renal fibrosis and inflammation via the TGF-β1/SMADS/AMPK/NF-κB pathways. BMC Complement Altern Med. 19:14. DOI: 10.1186/s12906-018-2410-7. PMID: 30630477. PMCID: PMC6327481.
Article30. Su SC, Hung YJ, Huang CL, Shieh YS, Chien CY, Chiang CF, Liu JS, Lu CH, Hsieh CH, Lin CM, Lee CH. 2019; Cilostazol inhibits hyperglucose-induced vascular smooth muscle cell dysfunction by modulating the RAGE/ERK/NF-κB signaling pathways. J Biomed Sci. 26:68. DOI: 10.1186/s12929-019-0550-9. PMID: 31492153. PMCID: PMC6731603.
Article31. Aghadavod E, Khodadadi S, Baradaran A, Nasri P, Bahmani M, Rafieian-Kopaei M. 2016; Role of oxidative stress and inflammatory factors in diabetic kidney disease. Iran J Kidney Dis. 10:337–343. PMID: 27903991.32. Rani V, Deep G, Singh RK, Palle K, Yadav UC. 2016; Oxidative stress and metabolic disorders: pathogenesis and therapeutic strategies. Life Sci. 148:183–193. DOI: 10.1016/j.lfs.2016.02.002. PMID: 26851532.
Article33. de Cavanagh EM, Toblli JE, Ferder L, Piotrkowski B, Stella I, Inserra F. 2006; Renal mitochondrial dysfunction in spontaneously hypertensive rats is attenuated by losartan but not by amlodipine. Am J Physiol Regul Integr Comp Physiol. 290:R1616–R1625. DOI: 10.1152/ajpregu.00615.2005. PMID: 16410402.
Article34. Domingueti CP, Dusse LM, Carvalho M, de Sousa LP, Gomes KB, Fernandes AP. 2016; Diabetes mellitus: the linkage between oxidative stress, inflammation, hypercoagulability and vascular complications. J Diabetes Complications. 30:738–745. DOI: 10.1016/j.jdiacomp.2015.12.018. PMID: 26781070.
Article35. Sesaki H, Jensen RE. 1999; Division versus fusion: Dnm1p and Fzo1p antagonistically regulate mitochondrial shape. J Cell Biol. 147:699–706. DOI: 10.1083/jcb.147.4.699. PMID: 10562274. PMCID: PMC2156171.
Article36. Chen H, Chan DC. 2015; Emerging functions of mammalian mitochondrial fusion and fission. Hum Mol Genet. 14 Spec No. 2:R283–R289. DOI: 10.1093/hmg/ddi270. PMID: 16244327.
Article37. Yoon Y. 2004; Sharpening the scissors: mitochondrial fission with aid. Cell Biochem Biophys. 41:193–206. DOI: 10.1385/CBB:41:2:193.
Article38. Yoon Y. 2005; Regulation of mitochondrial dynamics: another process modulated by Ca2+ signals? Sci STKE. 2005:pe18. DOI: 10.1126/stke.2802005pe18. PMID: 15840838.39. Riva A, Tandler B, Loffredo F, Vazquez E, Hoppel C. 2005; Structural differences in two biochemically defined populations of cardiac mitochondria. Am J Physiol Heart Circ Physiol. 289:H868–H872. DOI: 10.1152/ajpheart.00866.2004. PMID: 15821034.
Article40. Skulachev VP. 2001; Mitochondrial filaments and clusters as intracellular power-transmitting cables. Trends Biochem Sci. 26:23–29. DOI: 10.1016/S0968-0004(00)01735-7. PMID: 11165513.
Article41. Westermann B. 2002; Merging mitochondria matters: cellular role and molecular machinery of mitochondrial fusion. EMBO Rep. 3:527–531. DOI: 10.1093/embo-reports/kvf113. PMID: 12052774. PMCID: PMC1084147.42. Vanhorebeek I, De Vos R, Mesotten D, Wouters PJ, De Wolf-Peeters C, Van den Berghe G. 2005; Protection of hepatocyte mitochondrial ultrastructure and function by strict blood glucose control with insulin in critically ill patients. Lancet. 365:53–59. DOI: 10.1016/S0140-6736(04)17665-4. PMID: 15639679.
Article43. Kelley DE, He J, Menshikova EV, Ritov VB. 2002; Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes. 51:2944–2950. DOI: 10.2337/diabetes.51.10.2944. PMID: 12351431.
Article44. Senefeld J, Hunter SK. 2016; Molecular underpinnings of diabetic polyneuropathy. J Appl Physiol. 121:360. DOI: 10.1152/japplphysiol.00380.2016. PMID: 27451278.
Article45. Thanabalasingham G, Owen KR. 2011; Diagnosis and management of maturity onset diabetes of the young (MODY). BMJ. 343:d6044. DOI: 10.1136/bmj.d6044. PMID: 22012810.
Article46. Gutiérrez G, Mendoza C, Montaño LF, López-Marure R. 2007; Ceramide induces early and late apoptosis in human papilloma virus+ cervical cancer cells by inhibiting reactive oxygen species decay, diminishing the intracellular concentration of glutathione and increasing nuclear factor-kappaB translocation. Anticancer Drugs. 18:149–159. DOI: 10.1097/CAD.0b013e3280115111. PMID: 17159601.47. Larsen NB, Rasmussen M, Rasmussen LJ. 2005; Nuclear and mitochondrial DNA repair: similar pathways? Mitochondrion. 5:89–108. DOI: 10.1016/j.mito.2005.02.002. PMID: 16050976.
Article48. Kim MM, Clinger JD, Masayesva BG, Ha PK, Zahurak ML, Westra WH, Califano JA. 2004; Mitochondrial DNA quantity increases with histopathologic grade in premalignant and malignant head and neck lesions. Clin Cancer Res. 10:8512–8515. DOI: 10.1158/1078-0432.CCR-04-0734. PMID: 15623632.
Article49. Kang BP, Frencher S, Reddy V, Kessler A, Malhotra A, Meggs LG. 2003; High glucose promotes mesangial cell apoptosis by oxidant-dependent mechanism. Am J Physiol Renal Physiol. 284:F455–F466. DOI: 10.1152/ajprenal.00137.2002. PMID: 12419773.50. Wu Y, Qian Z, Fu S, Yue Y, Li Y, Sun R, Huang B, Yang D. 2018; IcarisideII improves left ventricular remodeling in spontaneously hypertensive rats by inhibiting the ASK1-JNK/p38 signaling pathway. Eur J Pharmacol. 819:68–79. DOI: 10.1016/j.ejphar.2017.11.035. PMID: 29175071.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Apoptosis and Ultrastructural Changes of Glomerular Endothelial Cells of Mice with Streptozotocin-induced Diabetic Nephropathy
- Observation of neutrophil extracellular traps in the development of diabetic nephropathy using diabetic murine models
- Blockade of Oxidative Stress by Vitamin C Ameliorates Albuminuria and Renal Sclerosis in Experimental Diabetic Rats
- The Epidemiology of Diabetic Nephropathy
- Recent Updates on Diabetic Nephropathy