Seroprevalence of Anti-SARS-CoV-2 Antibodies among Outpatients in Southwestern Seoul, Korea
- Affiliations
-
- 1Division of Infectious Diseases, Department of Internal Medicine, Korea University Guro Hospital, Korea University College of Medicine, Seoul, Korea
- 2Division of Infectious Diseases, Department of Internal Medicine, Hallym University College of Medicine, Chuncheon, Korea
- 3Department of Laboratory Medicine, Hallym University College of Medicine, Chuncheon, Korea
- 4Department of Laboratory Medicine, Korea University Guro Hospital, Korea University College of Medicine, Seoul, Korea
- 5Korea National Institute of Health, Korea Centers for Disease Control and Prevention, Cheongju, Korea
- KMID: 2505601
- DOI: http://doi.org/10.3346/jkms.2020.35.e311
Abstract
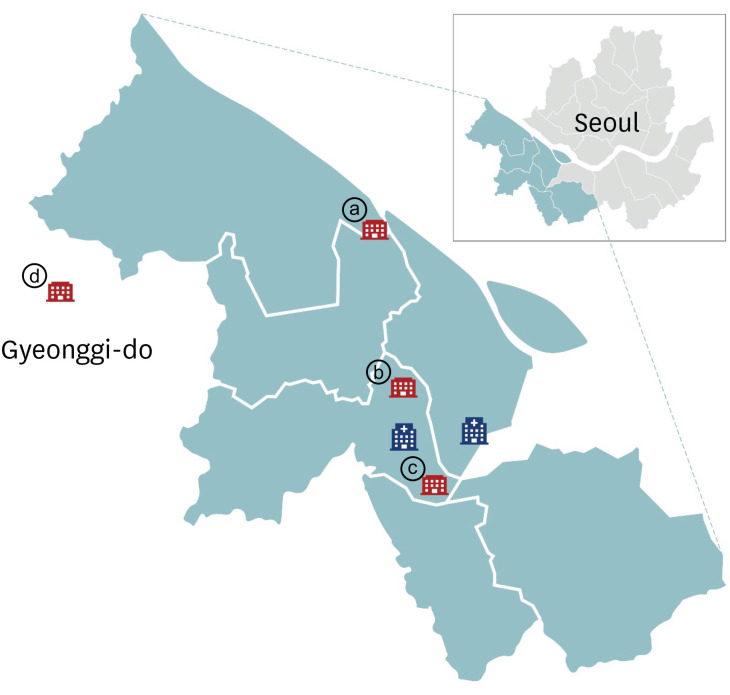
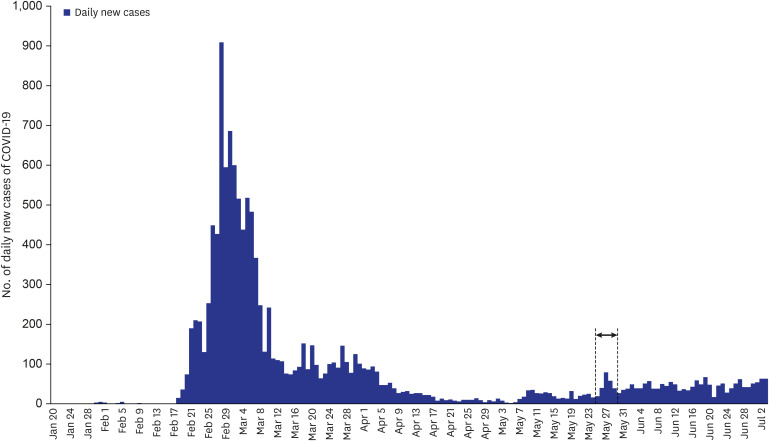
- Serosurveillance studies reveal the actual disease burden and herd immunity level in the population. In Seoul, Korea, a cross-sectional investigation showed 0.07% anti-severe acute respiratory syndrome coronavirus-2 antibody seropositivity among 1,500 outpatients of the university hospitals. Low seroprevalence reflects well-implemented social distancing. Serosurveillance should be repeated as the pandemic progresses.
Keyword
Figure
Cited by 4 articles
-
Comparison of Serologic Response of Hospitalized COVID-19 Patients Using 8 Immunoassays
Sun Min Lee, In-Suk Kim, Seungjin Lim, Su Jin Lee, Won-Joo Kim, Kyung-Hwa Shin, Soo Young Moon, Chulhun L. Chang
J Korean Med Sci. 2021;36(9):e64. doi: 10.3346/jkms.2021.36.e64.Developing a Framework for Pandemic COVID-19 Vaccine Allocation: a Modified Delphi Consensus Study in Korea
Min Joo Choi, Won Suk Choi, Hye Seong, Jun Yong Choi, Jong-Hyun Kim, Yae-Jean Kim, Eun Young Cho, Dong-Hyun Kim, Hyesook Park, Heeyoung Lee, Nam Joong Kim, Joon Young Song, Hee Jin Cheong, Sang Il Kim, Kyong Ran Peck
J Korean Med Sci. 2021;36(23):e166. doi: 10.3346/jkms.2021.36.e166.Severe Acute Respiratory Syndrome Coronavirus-2 Antibody Seropositivity among Healthcare Workers Working in Coronavirus Disease Wards
Seungjin Lim, Su Jin Lim, Sun Min Lee, In-Suk Kim, Taehwa Kim, Su Jin Lee
Korean J Healthc Assoc Infect Control Prev. 2021;26(1):24-30. doi: 10.14192/kjicp.2021.26.1.24.Performance Comparison of Five SARS-CoV-2 Antibody Assays for Seroprevalence Studies
Younhee Park, Ki Ho Hong, Su-Kyung Lee, Jungwon Hyun, Eun-Jee Oh, Jaehyeon Lee, Hyukmin Lee, Sang Hoon Song, Seung-Jung Kee, Gye Cheol Kwon, Su Hwan Kim, Hyeon-Nam Do, Ah-Ra Kim, June-Woo Lee, Sung Soon Kim, Hyun Soo Kim
Ann Lab Med. 2022;42(1):71-78. doi: 10.3343/alm.2022.42.1.71.
Reference
-
1. Zou L, Ruan F, Huang M, Liang L, Huang H, Hong Z, et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020; 382(12):1177–1179. PMID: 32074444.
Article2. Noh JY, Yoon JG, Seong H, Choi WS, Sohn JW, Cheong HJ, et al. Asymptomatic infection and atypical manifestations of COVID-19: comparison of viral shedding duration. J Infect. 2020; S0163-4453(20)30310-8.
Article3. Lee YH, Hong CM, Kim DH, Lee TH, Lee J. Clinical course of asymptomatic and mildly symptomatic patients with coronavirus disease admitted to community treatment centers, South Korea. Emerg Infect Dis. 2020; 26(10):
Article4. World Health Organization. Population-based age-stratified seroepidemiological investigation protocol for coronavirus 2019 (COVID-19) infection. Updated 2020. Accessed July 5, 2020. https://www.who.int/publications/i/item/WHO-2019-nCoV-Seroepidemiology-2020.2.5. Ministry of Health and Welfare (KR). Coronavirus disease-19, Republic of Korea. Updated 2020. Accessed July 5, 2020. http://ncov.mohw.go.kr/.6. Roche. Elecsys® Anti-SARS-CoV-2. Updated 2020. Accessed August 9, 2020. https://diagnostics.roche.com/global/en/products/params/elecsys-anti-sars-cov-2.html.7. Seoul Metropolitan Government. Statistics of registered foreigners in Seoul (by nationality/administrative district). Updated 2020. Accessed July 5, 2020. https://data.seoul.go.kr/dataList/803/S/2/datasetView.do?tab=C#.8. Seoul Metropolitan Government. Statistics of Seoul resident registered population (by administrative district). Updated 2020. Accessed August 9, 2020. https://data.seoul.go.kr/dataList/419/S/2/datasetView.do.9. Xu X, Sun J, Nie S, Li H, Kong Y, Liang M, et al. Seroprevalence of immunoglobulin M and G antibodies against SARS-CoV-2 in China. Nat Med. 2020; 26(8):1193–1195. PMID: 32504052.
Article10. Doi A, Iwata K, Kuroda H, Hasuike T, Nasu S, Kanda A, et al. Estimation of seroprevalence of novel coronavirus disease (COVID-19) using preserved serum at an outpatient setting in Kobe, Japan: a cross-sectional study. medRxiv. Updated 2020. Accessed July 7, 2020. DOI: 10.1101/2020.04.26.20079822.11. To KK, Cheng VC, Cai JP, Chan KH, Chen LL, Wong LH, et al. Seroprevalence of SARS-CoV-2 in Hong Kong and in residents evacuated from Hubei province, China: a multicohort study. Lancet Microbe. 2020; 1(3):E111–8.
Article12. Sood N, Simon P, Ebner P, Eichner D, Reynolds J, Bendavid E, et al. Seroprevalence of SARS-CoV-2-specific antibodies among adults in Los Angeles County, California, on April 10-11, 2020. JAMA. 2020; 323(23):2425–2427.
Article13. Percivalle E, Cambiè G, Cassaniti I, Nepita EV, Maserati R, Ferrari A, et al. Prevalence of SARS-CoV-2 specific neutralising antibodies in blood donors from the Lodi Red Zone in Lombardy, Italy, as at 06 April 2020. Euro Surveill. 2020; 25(24):2001031.
Article14. Stringhini S, Wisniak A, Piumatti G, Azman AS, Lauer SA, Baysson H, et al. Seroprevalence of anti-SARS-CoV-2 IgG antibodies in Geneva, Switzerland (SEROCoV-POP): a population-based study. Lancet. 2020; 396(10247):313–319. PMID: 32534626.
Article15. Slot E, Hogema BM, Reusken CB, Reimerink JH, Molier M, Karregat JH, IJlst J, et al. Herd immunity is not a realistic exit strategy during a COVID-19 outbreak. Res Sq. Updated 2020. Accessed July 7, 2020. DOI: 10.21203/rs.3.rs-25862/v1.16. Habib H. Has Sweden's controversial COVID-19 strategy been successful? BMJ. 2020; 369:m2376. PMID: 32532807.
Article17. Public Health Agency of Sweden. Första resultaten från pågående undersökning av antikroppar för COVID-19-virus. Updated 2020. Accessed July 7, 2020. https://www.folkhalsomyndigheten.se/nyheter-och-press/nyhetsarkiv/2020/maj/forsta-resultaten-fran-pagaende-undersokning-av-antikroppar-for-covid-19-virus/.18. Song SK, Lee DH, Nam JH, Kim KT, Do JS, Kang DW, et al. IgG Seroprevalence of COVID-19 among individuals without a history of the coronavirus disease infection in Daegu, Korea. J Korean Med Sci. 2020; 35(29):e269. PMID: 32715672.
Article19. Cheng MP, Yansouni CP, Basta NE, Desjardins M, Kanjilal S, Paquette K, et al. Serodiagnostics for severe acute respiratory syndrome-related coronavirus-2: a narrative review. Ann Intern Med. 2020; M20-2854.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Seroprevalence of antibodies to SARS-CoV-2 and predictors of seropositivity among employees of a teaching hospital in New Delhi, India
- The Seroprevalence of SARS-CoV-2 in Children During Early COVID-19 Pandemic in Korea: A Nationwide, Population-Based Study
- Seroprevalence of immunoglobulin G antibodies against SARS-CoV-2 in children and adolescents in Delhi, India, from January to October 2021: a repeated cross-sectional analysis
- Evaluation of Seroprevalence of SARS-CoV-2 IgG in Healthcare Workers in a Tertiary Hospital in Seoul
- Seroprevalence of SARS‑CoV‑2 antibodies in patients with hematological and oncological diseases in early 2024