J Korean Med Sci.
2022 Nov;37(44):e314. 10.3346/jkms.2022.37.e314.
The Seroprevalence of SARS-CoV-2 in Children During Early COVID-19 Pandemic in Korea: A Nationwide, Population-Based Study
- Affiliations
-
- 1Department of Pediatrics, Incheon St. Mary’s Hospital, The Catholic University of Korea, Incheon, Korea
- 2Department of Pediatrics, Korea University Anam Hospital, Seoul, Korea
- 3Department of Diagnostic Immunology, Seegene Medical Foundation, Seoul, Korea
- 4Department of Pediatrics, St. Vincent’s Hospital, The Catholic University of Korea, Suwon, Korea
- KMID: 2535180
- DOI: http://doi.org/10.3346/jkms.2022.37.e314
Abstract
- Background
Coronavirus disease 2019 (COVID-19) is often asymptomatic and associated with mild clinical symptoms in children. Social distancing measures have led to a relatively small number of confirmed COVID-19 cases in Korea than in other countries in the earlier pandemic phase. Previous seroprevalence studies in Korean adults before the introduction of COVID-19 vaccination campaign have shown a low antibody positivity rate. However, data on COVID-19 seroprevalence in Korean children remained scarce. In this study, we assessed the seroprevalence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in children in Korea.
Methods
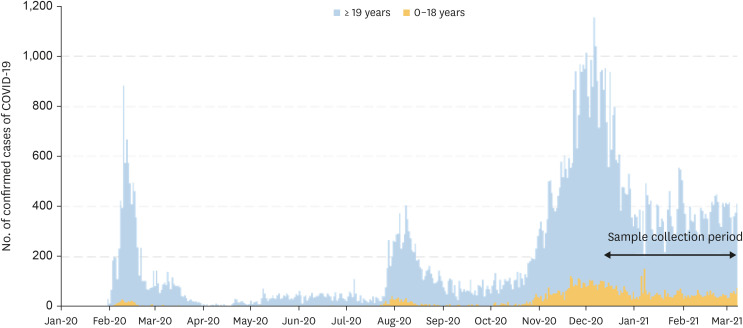
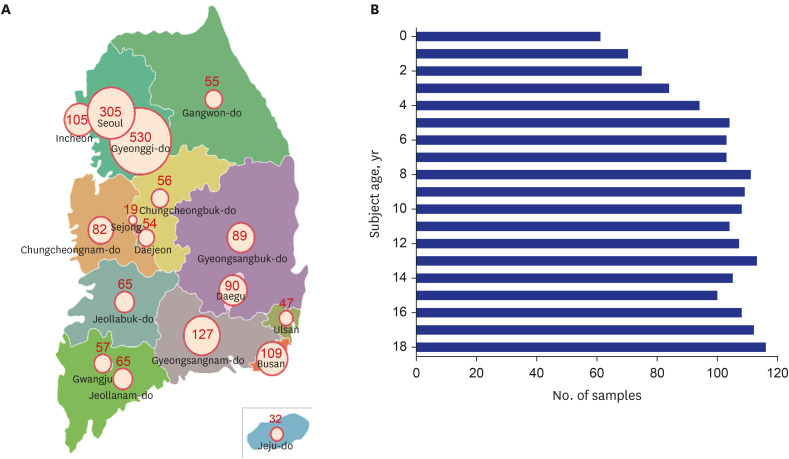
Between December 2020 and March 2021, stratified serum samples were collected from children aged 0–18 years in 17 different regions across the country. The SARS-CoV-2 antibody test was conducted using an electro-chemiluminescence immunoassay (ECLIA) to detect the antibodies against nucleocapsid antigens of SARS-CoV-2. Samples that tested positive using the ECLIA were reflexed to an additional plaque reduction neutralization test (PRNT) for SARS-CoV-2.
Results
A total of 1,887 samples were collected. Excluding 21 samples collected from regional medical centers, 1,866 samples were included in the final analysis. Two samples (0.11%) were positive for the antibodies against nucleocapsid antigens of SARS-CoV-2. Both samples were shown to have neutralizing antibodies for SARS-CoV-2 via PRNT.
Conclusion
After 1 year since the start of COVID-19 pandemic, the seroprevalence of SARSCoV-2 among Korean children was 0.11%, which was lower than the adults (0.52%) in another study conducted during a similar period. In the early stages of the COVID-19 pandemic, the seroprevalence of SARS-CoV-2 in Korea was lower than those of other countries, which was presumed to be the consequence of a very strong social distancing measures.
Keyword
Figure
Reference
-
1. Central Disaster Management Headquarters. Coronavirus (COVID-19), Republic of Korea. Updated 2021. Accessed June 19, 2022. http://ncov.mohw.go.kr/en/ .2. Centers for Disease Control and Prevention. COVID data tracker. Updated 2021. Accessed October 9, 2021. https://covid.cdc.gov/covid-data-tracker/#national-lab .3. Korea Disease Control and Prevention Agency. Updated 2021. Accessed November 5, 2021. https://www.kdca.go.kr/board/board.es?mid=a20501010000&bid=0015&list_no=712965&cg_code=&act=view&nPage=61 .4. Noh JY, Seo YB, Yoon JG, Seong H, Hyun H, Lee J, et al. Seroprevalence of anti-SARS-CoV-2 antibodies among outpatients in southwestern Seoul, Korea. J Korean Med Sci. 2020; 35(33):e311. PMID: 32830472.5. Jeong HW, Chang HH, Kim EJ, Kim YK, Kim SM, Kim EH, et al. Differences in seroprevalence between epicenter and non-epicenter areas of the COVID-19 outbreak in South Korea. J Microbiol. 2021; 59(5):530–533. PMID: 33907974.6. Nah EH, Cho S, Park H, Hwang I, Cho HI. Nationwide seroprevalence of antibodies to SARS-CoV-2 in asymptomatic population in South Korea: a cross-sectional study. BMJ Open. 2021; 11(4):e049837.7. Ministry of the Interior and Safety. Updated 2021. Accessed October 21, 2021. https://jumin.mois.go.kr/ .8. Roche Diagnostics. Elecsys® Anti-SARS-CoV-2, Immunoassay for the qualitative detection of antibodies (incl. IgG) against SARS-CoV-2. Elecsys® Anti-SARS-CoV-2. Updated 2021. Accessed November 5, 2021. https://www.roche.com/ .9. Grist NR, Ross CA, Bell EJ. Diagnostic Methods in Clinical Virology. 2nd ed. Philadelphia, PA, USA: J.B. Lippincott & Co;1974.10. Ministry of Culture. Sports and tourism. Republic of Korea. Updated 2021. Accessed October 21, 2021. https://www.korea.kr/special/policyFocusView.do?newsId=148888045&pkgId=49500742 .11. Waterfield T, Watson C, Moore R, Ferris K, Tonry C, Watt A, et al. Seroprevalence of SARS-CoV-2 antibodies in children: a prospective multicentre cohort study. Arch Dis Child. 2021; 106(7):680–686. PMID: 33172887.12. Tönshoff B, Müller B, Elling R, Renk H, Meissner P, Hengel H, et al. Prevalence of SARS-CoV-2 infection in children and their parents in Southwest Germany. JAMA Pediatr. 2021; 175(6):586–593. PMID: 33480966.13. Torres JP, Piñera C, De La Maza V, Lagomarcino AJ, Simian D, Torres B, et al. Severe acute respiratory syndrome coronavirus 2 antibody prevalence in blood in a large school community subject to a coronavirus disease 2019 outbreak: a cross-sectional study. Clin Infect Dis. 2021; 73(2):e458–e465. PMID: 32649743.14. Rostami A, Sepidarkish M, Leeflang MM, Riahi SM, Nourollahpour Shiadeh M, Esfandyari S, et al. SARS-CoV-2 seroprevalence worldwide: a systematic review and meta-analysis. Clin Microbiol Infect. 2021; 27(3):331–340. PMID: 33228974.15. Ministry of Culture. Sports and tourism, Republic of Korea. Updated 2021. Accessed November 5, 2021. https://www.korea.kr/archive/speechView.do?newsId=132033265 .16. UNESCO. Updated 2021. Accessed November 5, 2021. https://en.unesco.org/covid19/educationresponse .17. OECD Library. The State of Global Education. Updated 2021. Accessed November 5, 2021. https://www.oecd-ilibrary.org/education/the-state-of-global-education_1a23bb23-en .18. Milani GP, Dioni L, Favero C, Cantone L, Macchi C, Delbue S, et al. Serological follow-up of SARS-CoV-2 asymptomatic subjects. Sci Rep. 2020; 10(1):20048. PMID: 33208819.19. Petersen LR, Sami S, Vuong N, Pathela P, Weiss D, Morgenthau BM, et al. Lack of antibodies to SARS-CoV-2 in a large cohort of previously infected persons. Clin Infect Dis. 2021; 73(9):e3066–e3073. PMID: 33147319.20. Kaufman HW, Chen Z, Meyer WA 3rd, Wohlgemuth JG. Insights from patterns of SARS-CoV-2 immunoglobulin G serology test results from a national clinical laboratory, United States, March-July 2020. Popul Health Manag. 2021; 24(S1):S35–S42. PMID: 33216694.21. Centers for Disease Control and Prevention. Interim guidelines for COVID-19 antibody testing. Updated 2022. Accessed June 3, 2022. https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/antibody-tests-guidelines.html .22. Our World in Data. Daily new confirmed COVID-19 cases per million people. Updated 2022. Accessed August 17, 2022. https://ourworldindata.org/explorers/coronavirus-data-explorer?zoomToSelection=true&time=2022-01-13.2022-06-04&facet=none&pickerSort=desc&pickerMetric=new_cases_smoothed&Metric=Confirmed+cases&Interval=New+per+day&Relative+to+Population=true&Color+by+test+positivity=false&country=USA~ITA~CAN~DEU~GBR~FRA~JPN~SLE~Europe~OWID_WRL~GIN~KOR~South+America~Asia~North+America~Oceania .23. Korea Disease Control and Prevention Agency. Updated 2022. Accessed August 17, 2022. https://www.kdca.go.kr/board/board.es?mid=a20501010000&bid=0015&list_no=719440&cg_code=&act=view&nPage=25 .24. Central Disaster Management Headquarters. Coronavirus (COVID-19), Republic of Korea. Updated 2022. Accessed August 17, 2022. http://ncov.mohw.go.kr/en/bdBoardList.do?brdId=16&brdGubun=163&dataGubun=&ncvContSeq=&contSeq=&board_id=&gubun= .
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- COVID-19 and diabetes in children
- Clinical implications of coronavirus disease 2019 in neonates
- Age-Related Morbidity and Mortality among Patients with COVID-19
- Evaluation of Seroprevalence of SARS-CoV-2 IgG in Healthcare Workers in a Tertiary Hospital in Seoul
- Epidemiology, Virology, and Clinical Features of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2; Coronavirus Disease-19)