Ann Surg Treat Res.
2020 Sep;99(3):161-170. 10.4174/astr.2020.99.3.161.
The prognostic significance of serum lactate dehydrogenase-to-albumin ratio in colorectal cancer
- Affiliations
-
- 1Department of Gastrointestinal Surgery, Dicle University Faculty of Medicine, Diyarbakır, Turkey
- 2Department of Surgery, Elazığ Training and Research Hospital, University of Health Sciences, Elazığ, Turkey
- 3Department of Pharmacology, Dicle University Faculty of Medicine, Diyarbakır, Turkey
- KMID: 2505489
- DOI: http://doi.org/10.4174/astr.2020.99.3.161
Abstract
- Purpose
The purpose of our study was initially to explore the prognostic role of LDH-to-albumin ratio in patients with colorectal carcinoma (CRC) undergoing curative resection.
Methods
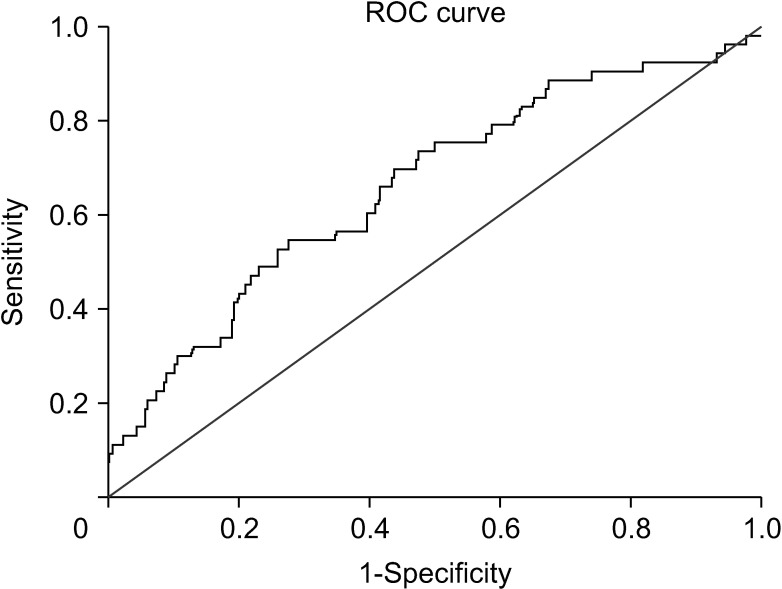
The retrospective study included 295 CRC patients that underwent curative resection. According to timedependent receiver operating characteristics (ROC) analysis, the optimal cutoff value for pretreatment LDH-to-albumin ratio was 52.7. Cox regression univariate and multivariate analyses were utilized to analyze the prognostic factors for disease-free survival (DFS) and overall survival (OS).
Results
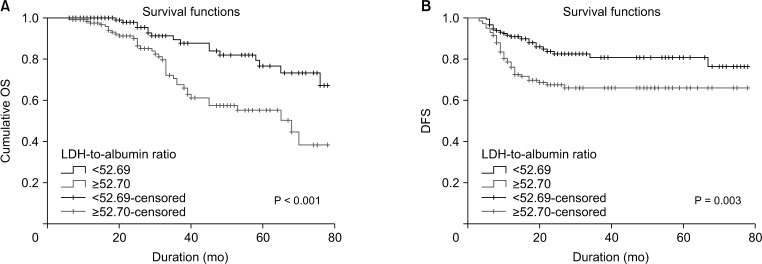
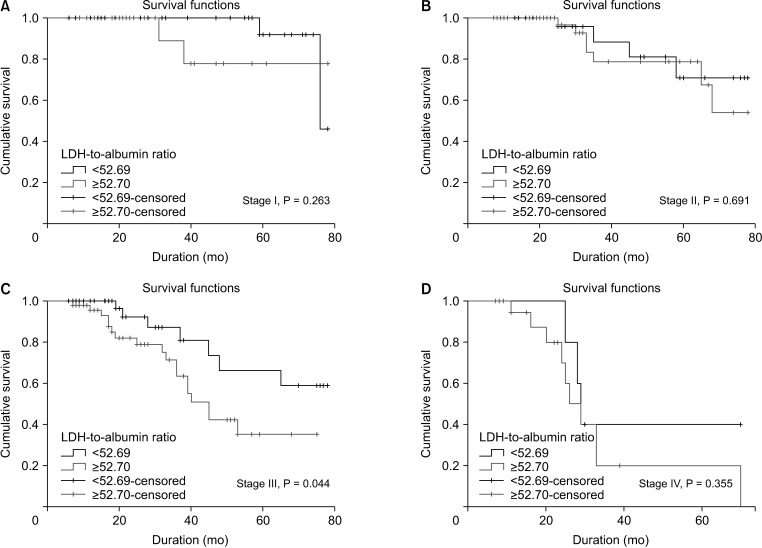
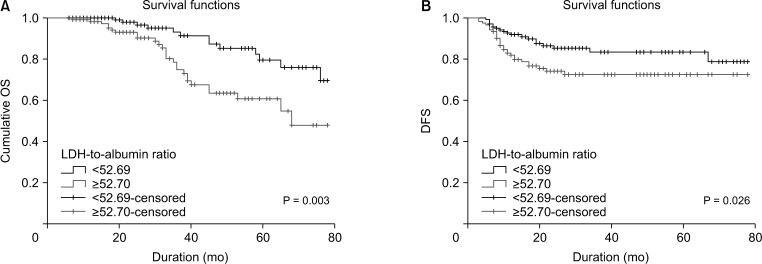
The 295 participants included 117 women (39.7%) and had an overall mean age of 55.8 ± 14.1 years. The median follow-up period was 31.8 ± 21 months (range, 6–78 months) and 53 patients (18.0%) died from cancer during the followup period. The 5-year DFS and OS rates were 65.4% and 68.5% in patients with LDH-to-albumin ratio <52.7 (n = 152), and were 55.2% and 55.4% in patients with LDH-to-albumin ratio ≥52.7 (n = 143), respectively. Kaplan-Meier curves showed that LDH-to-albumin ratio ≥52.7 was significantly associated with worse DFS and OS (p = 0.003 and p < 0.001, respectively). Multivariate analyses revealed that LDH-to-albumin ratio was an independent predictor of resectable CRC (odds ratio, 2.104; 95% confidence interval, 1.112–3.982; p = 0.022).
Conclusion
Our study revealed that high pretreatment LDH-to-albumin ratio level was an unfavorable prognosticator in patients with CRC undergoing curative resection. LDH-to-albumin ratio is a candidate to be a prognostic biomarker in clinical practice.
Figure
Reference
-
1. Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017; 66:683–691. PMID: 26818619.2. Guraya SY. Pattern, stage, and time of recurrent colorectal cancer after curative surgery. Clin Colorectal Cancer. 2019; 18:e223–e228. PMID: 30792036.3. Rossi S, Basso M, Strippoli A, Schinzari G, D'Argento E, Larocca M, et al. Are markers of systemic inflammation good prognostic indicators in colorectal cancer? Clin Colorectal Cancer. 2017; 16:264–274. PMID: 28412137.4. Park JH, Ishizuka M, McSorley ST, Kubota K, Roxburgh CS, Nagata H, et al. Staging the tumor and staging the host: a two centre, two country comparison of systemic inflammatory responses of patients undergoing resection of primary operable colorectal cancer. Am J Surg. 2018; 216:458–464. PMID: 28967380.5. Wang F, He W, Jiang C, Guo G, Ke B, Dai Q, et al. Prognostic value of inflammation-based scores in patients receiving radical resection for colorectal cancer. BMC Cancer. 2018; 18:1102. PMID: 30419863.6. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008; 454:436–444. PMID: 18650914.7. Shibutani M, Maeda K, Nagahara H, Iseki Y, Ikeya T, Hirakawa K. Prognostic signi f icance of the preoperat ive lymphocyte-to-monocyte ratio in patients with colorectal cancer. Oncol Lett. 2017; 13:1000–1006. PMID: 28356991.8. Zha X, Wang F, Wang Y, He S, Jing Y, Wu X, et al. Lactate dehydrogenase B is critical for hyperactive mTOR-mediated tumorigenesis. Cancer Res. 2011; 71:13–18. PMID: 21199794.9. Le A, Cooper CR, Gouw AM, Dinavahi R, Maitra A, Deck LM, et al. Inhibition of lactate dehydrogenase A induces oxidative stress and inhibits tumor progression. Proc Natl Acad Sci U S A. 2010; 107:2037–2042. PMID: 20133848.10. Petrelli F, Cabiddu M, Coinu A, Borgonovo K, Ghilardi M, Lonati V, et al. Prognostic role of lactate dehydrogenase in solid tumors: a systematic review and meta-analysis of 76 studies. Acta Oncol. 2015; 54:961–970. PMID: 25984930.11. Wei Y, Xu H, Dai J, Peng J, Wang W, Xia L, et al. Prognostic significance of serum lactic acid, lactate dehydrogenase, and albumin levels in patients with metastatic colorectal cancer. Biomed Res Int. 2018; 2018:1804086. PMID: 30627541.12. Yu SL, Xu LT, Qi Q, Geng YW, Chen H, Meng ZQ, et al. Serum lactate dehydrogenase predicts prognosis and correlates with systemic inflammatory response in patients with advanced pancreatic cancer after gemcitabine-based chemotherapy. Sci Rep. 2017; 7:45194. PMID: 28345594.13. Pence HH, Gundes E, Senger AS, Alsaadoni H, Ciyiltepe H, Gulmez S, et al. Prognostic significance of preoperative serum lactate dehydrogenase in patients with resectable gastric adenocarcinoma. Int J Clin Exp Med. 2019; 12:2746–2752.14. Chen ZH, Qiu MZ, Wu XY, Wu QN, Lu JH, Zeng ZL, et al. Elevated baseline serum lactate dehydrogenase indicates a poor prognosis in primary duodenum adenocarcinoma patients. J Cancer. 2018; 9:512–520. PMID: 29483956.15. Li MX, Zhao H, Bi XY, Li ZY, Yao XS, Li H, et al. Lactate dehydrogenase is a prognostic indicator in patients with hepatocellular carcinoma treated by sorafenib: results from the real life practice in HBV endemic area. Oncotarget. 2016; 7:86630–86647. PMID: 27880930.16. Gan J, Wang W, Yang Z, Pan J, Zheng L, Yin L. Prognostic value of pretreatment serum lactate dehydrogenase level in pancreatic cancer patients: a meta-analysis of 18 observational studies. Medicine (Baltimore). 2018; 97:e13151. PMID: 30431587.17. Guo HW, Yuan TZ, Chen JX, Zheng Y. Prognostic value of pretreatment albumin/globulin ratio in digestive system cancers: a meta-analysis. PLoS One. 2018; 13:e0189839. PMID: 29300750.18. Fujii T, Sutoh T, Morita H, Katoh T, Yajima R, Tsutsumi S, et al. Serum albumin is superior to prealbumin for predicting short-term recurrence in patients with operable colorectal cancer. Nutr Cancer. 2012; 64:1169–1173. PMID: 23163845.19. Boonpipattanapong T, Chewatanakornkul S. Preoperative carcinoembryonic antigen and albumin in predicting survival in patients with colon and rectal carcinomas. J Clin Gastroenterol. 2006; 40:592–595. PMID: 16917399.20. Feng JF, Wang L, Yang X, Jiang YH. Prognostic value of lactate dehydrogenase to albumin ratio (LAR) in patients with resectable esophageal squamous cell carcinoma. Cancer Manag Res. 2019; 11:7243–7251. PMID: 31447584.21. Gan W, Zhang MX, Wang JX, Fu YP, Huang JL, Yi Y, et al. Prognostic impact of lactic dehydrogenase to albumin ratio in hepatocellular carcinoma patients with Child-Pugh I who underwent curative resection: a prognostic nomogram study. Cancer Manag Res. 2018; 10:5383–5394. PMID: 30464634.22. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004; 240:205–213. PMID: 15273542.23. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene F, Trotti A. AJCC Cancer Staging Handbook. 7th ed. New York: Springer;2010.24. Zhou W, Capello M, Fredolini C, Racanicchi L, Piemonti L, Liotta LA, et al. MS analysis reveals O-methylation of L - lactate dehydrogenase from pancreatic ductal adenocarcinoma cells. Electrophoresis. 2012; 33:1850–1854. PMID: 22740473.25. Ding J, Karp JE, Emadi A. Elevated lactate dehydrogenase (LDH) can be a marker of immune suppression in cancer: interplay between hematologic and solid neoplastic clones and their microenvironments. Cancer Biomark. 2017; 19:353–363. PMID: 28582845.26. McMillan DC, Watson WS, O'Gorman P, Preston T, Scott HR, McArdle CS. Albumin concentrations are primarily determined by the body cell mass and the systemic inf lammator y response in cancer patients with weight loss. Nutr Cancer. 2001; 39:210–213. PMID: 11759282.27. Hardt J, Pilz L, Magdeburg J, Kienle P, Post S, Magdeburg R. Preoperative hypoalbuminemia is an independent risk factor for increased high-grade morbidity after elective rectal cancer resection. Int J Colorectal Dis. 2017; 32:1439–1446. PMID: 28823064.28. Truong A, Hanna MH, Moghadamyeghaneh Z, Stamos MJ. Implications of preoperative hypoalbuminemia in colorectal surgery. World J Gastrointest Surg. 2016; 8:353–362. PMID: 27231513.29. Li G, Wang Z, Xu J, Wu H, Cai S, He Y. The prognostic value of lactate dehydrogenase levels in colorectal cancer: a meta-analysis. BMC Cancer. 2016; 16:249. PMID: 27016045.30. Silvestris N, Scartozzi M, Graziano G, Santini D, Lorusso V, Maiello E, et al. Basal and bevacizumab-based therapy-induced changes of lactate dehydrogenases and fibrinogen levels and clinical outcome of previously untreated metastatic colorectal cancer patients: a multicentric retrospective analysis. Expert Opin Biol Ther. 2015; 15:155–162. PMID: 25411089.31. Hackl C, Neumann P, Gerken M, Loss M, Klinkhammer-Schalke M, Schlitt HJ. Treatment of colorectal liver metastases in Germany: a ten-year population-based analysis of 5772 cases of primary colorectal adenocarcinoma. BMC Cancer. 2014; 14:810. PMID: 25369977.32. Kim YI, Park IJ, Kim JE, Kim SY, Park JH, Lee JH, et al. Hepatic resection after neoadjuvant chemotherapy for patients with liver metastases from colorectal cancer: need for cautious planning. Ann Surg Treat Res. 2019; 97:245–253. PMID: 31742209.33. Vigano L, Capussotti L, Lapointe R, Barroso E, Hubert C, Giuliante F, et al. Early recurrence after liver resection for colorectal metastases: risk factors, prognosis, and treatment: a LiverMetSurvey-based study of 6,025 patients. Ann Surg Oncol. 2014; 21:1276–1286. PMID: 24346766.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- An enzymatic method for the detection of human serum albumin
- A study on the serum and cell lactate dehydrogenase isoenzyme in hematologic malignancies
- Prognostic Factors for Survival in Patients with Stage IV Non-small Cell Lung Cancer
- Follicular Lactate Dehydrogenase Activity and Steroid Concentrations in the Immature Gilt Ovary
- Inflammation-based score (Glasgow prognostic score) as an independent prognostic factor in colorectal cancer patients