Intest Res.
2020 Jul;18(3):325-336. 10.5217/ir.2019.00093.
Melatonin in the colon modulates intestinal microbiota in response to stress and sleep deprivation
- Affiliations
-
- 1Division of Gastroenterology, Department of Internal Medicine, Nowon Eulji Medical Center, Eulji University School of Medicine, Seoul, Korea
- 2Department of Health, Environment and Safety, Graduate School of Health Science, Eulji University, Seongnam, Korea
- 3Departemnt of Biomedical Laboratory Science, Graduate School of Health Science, Eulji University, Seongnam, Korea
- 4Department of Biomedical Laboratory Science, Eulji University, Seongnam, Korea
- 5Department of Pathology, Eulji University School of Medicine, Seoul, Korea
- KMID: 2504588
- DOI: http://doi.org/10.5217/ir.2019.00093
Abstract
- Background/Aims
Stress is closely related to the deterioration of digestive disease. Melatonin has potent anti-inflammatory properties. The objective of this study was to determine the effect of water stress (WS) and sleep deprivation (SD) on intestinal microbiota and roles of melatonin in stressful condition.
Methods
We used C57BL/6 mice and specially designed water bath for stress and SD for 10 days. We measured melatonin concentrations in serum, feces, and colon tissues by high-performance liquid chromatography. Genomic DNA was extracted from feces and amplified using primers targeting V3 to V4 regions of bacterial 16S ribosomal RNA genes.
Results
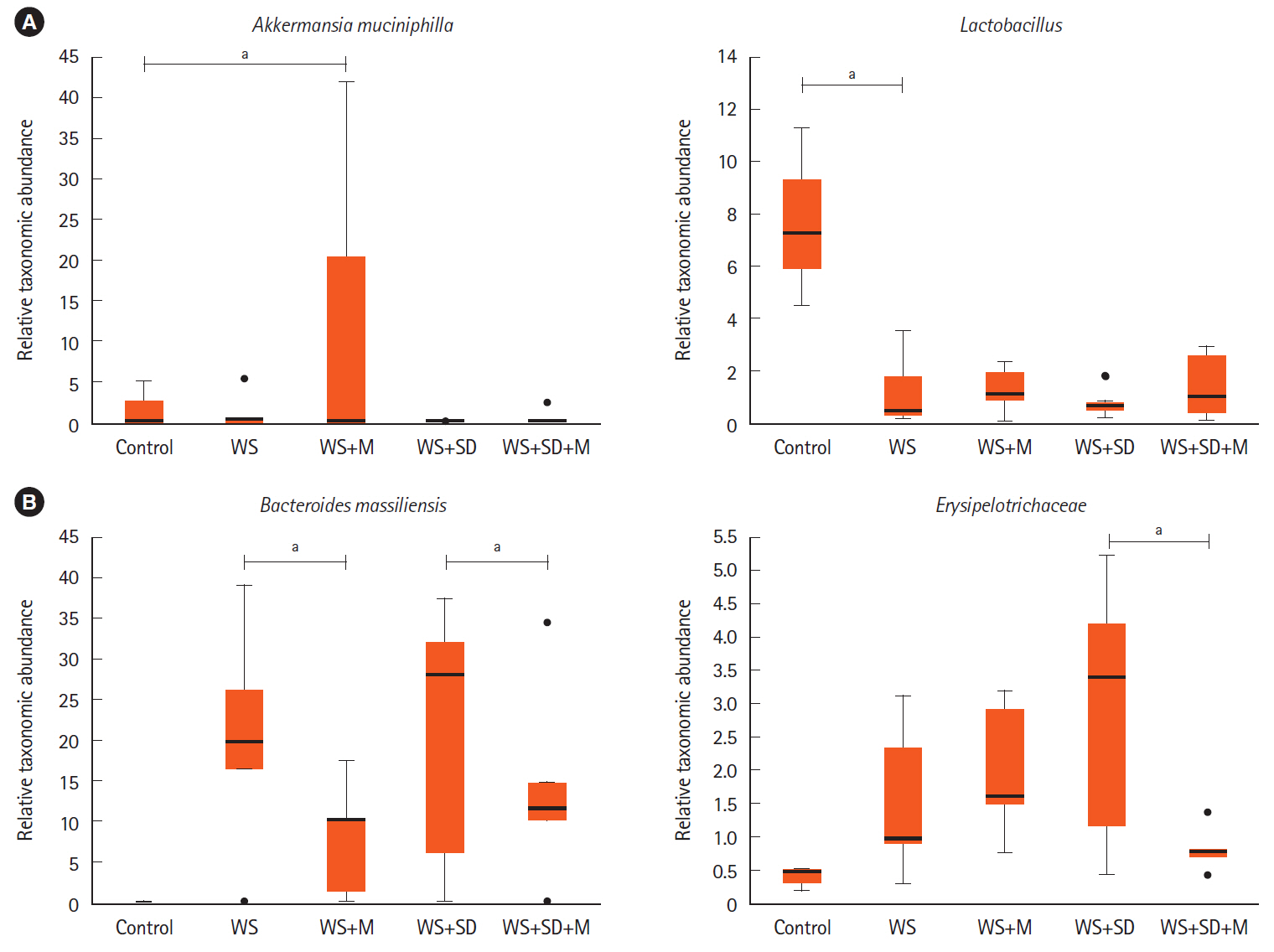
Compared to the control, melatonin concentration was lower in the WS and SD. Fecal concentration was 0.132 pg/mL in control, 0.062 pg/mL in WS, and 0.068 pg/mL in SD. In colon tissue, it was 0.45 pg/mL in control, 0.007 pg/mL in WS, and 0.03 pg/mL in SD. After melatonin treatment, melatonin concentrations in feces and colon tissue were recovered to the level of control. Metagenomic analysis of microbiota showed abundance in colitogenic microbiota in WS and SD. Melatonin injection attenuated this harmful effect. WS and SD showed decreased Lactobacillales and increased Erysipelotrichales and Enterobacteriales. Melatonin treatment increased Akkermansia muciniphila and Lactobacillus and decreased Bacteroides massiliensis and Erysipelotrichaceae.
Conclusions
This study showed that stress and SD could affect intestinal dysbiosis and increase colitogenic microbiota, which could contribute to the aggravating digestive disease. Melatonin concentrations in feces and colon tissue decreased under WS and SD. Melatonin treatment brought recovery of melatonin concentration in colon tissue and modulating dysbiosis of intestinal microbiota.
Figure
Reference
-
1. Jostins L, Ripke S, Weersma RK, et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012; 491:119–124.2. Sonnenberg A. Occupational distribution of inflammatory bowel disease among German employees. Gut. 1990; 31:1037–1040.
Article3. Ranjbaran Z, Keefer L, Farhadi A, Stepanski E, Sedghi S, Keshavarzian A. Impact of sleep disturbances in inflammatory bowel disease. J Gastroenterol Hepatol. 2007; 22:1748–1753.
Article4. Swanson GR, Burgess HJ, Keshavarzian A. Sleep disturbances and inflammatory bowel disease: a potential trigger for disease flare? Expert Rev Clin Immunol. 2011; 7:29–36.
Article5. Nishida A, Inoue R, Inatomi O, Bamba S, Naito Y, Andoh A. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin J Gastroenterol. 2018; 11:1–10.
Article6. Chung SH, Park YS, Kim OS, et al. Melatonin attenuates dextran sodium sulfate induced colitis with sleep deprivation: possible mechanism by microarray analysis. Dig Dis Sci. 2014; 59:1134–1141.
Article7. Kim TK, Park YS, Baik HW, et al. Melatonin modulates adiponectin expression on murine colitis with sleep deprivation. World J Gastroenterol. 2016; 22:7559–7568.
Article8. Park YS, Chung SH, Lee SK, et al. Melatonin improves experimental colitis with sleep deprivation. Int J Mol Med. 2015; 35:979–986.
Article9. Paulose JK, Wright JM, Patel AG, Cassone VM. Human gut bacteria are sensitive to melatonin and express endogenous circadian rhythmicity. PLoS One. 2016; 11:e0146643.
Article10. Xu P, Wang J, Hong F, et al. Melatonin prevents obesity through modulation of gut microbiota in mice. J Pineal Res. 2017; 62:e12399.
Article11. Reiter RJ, Mayo JC, Tan DX, Sainz RM, Alatorre-Jimenez M, Qin L. Melatonin as an antioxidant: under promises but over delivers. J Pineal Res. 2016; 61:253–278.
Article12. Reiter RJ, Tan DX, Fuentes-Broto L. Melatonin: a multitasking molecule. Prog Brain Res. 2010; 181:127–151.
Article13. Ren W, Liu G, Chen S, et al. Melatonin signaling in T cells: functions and applications. J Pineal Res. 2017; 62:e12394.
Article14. Marquez E, Sánchez-Fidalgo S, Calvo JR, la de Lastra CA, Motilva V. Acutely administered melatonin is beneficial while chronic melatonin treatment aggravates the evolution of TNBS-induced colitis. J Pineal Res. 2006; 40:48–55.
Article15. Bubenik GA, Brown GM. Pinealectomy reduces melatonin levels in the serum but not in the gastrointestinal tract of rats. Biol Signals. 1997; 6:40–44.
Article16. Chen CQ, Fichna J, Bashashati M, Li YY, Storr M. Distribution, function and physiological role of melatonin in the lower gut. World J Gastroenterol. 2011; 17:3888–3898.
Article17. Necefli A, Tulumoğlu B, Giriş M, et al. The effect of melatonin on TNBS-induced colitis. Dig Dis Sci. 2006; 51:1538–1545.
Article18. Swanson GR, Gorenz A, Shaikh M, et al. Decreased melatonin secretion is associated with increased intestinal permeability and marker of endotoxemia in alcoholics. Am J Physiol Gastrointest Liver Physiol. 2015; 308:G1004–G1011.
Article19. Fernández-Gil B, Moneim AE, Ortiz F, et al. Melatonin protects rats from radiotherapy-induced small intestine toxicity. PLoS One. 2017; 12:e0174474.
Article20. Bernstein CN. The brain-gut axis and stress in inflammatory bowel disease. Gastroenterol Clin North Am. 2017; 46:839–846.
Article21. Fernandez-Mendoza J, Baker JH, Vgontzas AN, Gaines J, Liao D, Bixler EO. Insomnia symptoms with objective short sleep duration are associated with systemic inflammation in adolescents. Brain Behav Immun. 2017; 61:110–116.
Article22. Irwin MR, Opp MR. Sleep health: reciprocal regulation of sleep and innate immunity. Neuropsychopharmacology. 2017; 42:129–155.
Article23. Legaki E, Gazouli M. Influence of environmental factors in the development of inflammatory bowel diseases. World J Gastrointest Pharmacol Ther. 2016; 7:112–125.
Article24. Pirinen T, Kolho KL, Ashorn M, Aronen ET. Sleep and emotional and behavioral symptoms in adolescents with inflammatory bowel disease. Sleep Disord. 2014; 2014:379450.
Article25. Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009; 9:313–323.
Article26. Kamada N, Núñez G. Regulation of the immune system by the resident intestinal bacteria. Gastroenterology. 2014; 146:1477–1488.
Article27. Buffie CG, Pamer EG. Microbiota-mediated colonization resistance against intestinal pathogens. Nat Rev Immunol. 2013; 13:790–801.
Article28. Shang Q, Sun W, Shan X, et al. Carrageenan-induced colitis is associated with decreased population of anti-inflammatory bacterium, Akkermansia muciniphila, in the gut microbiota of C57BL/6J mice. Toxicol Lett. 2017; 279:87–95.
Article29. Derrien M, Belzer C, de Vos WM. Akkermansia muciniphila and its role in regulating host functions. Microb Pathog. 2017; 106:171–181.
Article30. Boureau H, Decré D, Carlier JP, Guichet C, Bourlioux P. Identification of a Clostridium cocleatum strain involved in an antiClostridium difficile barrier effect and determination of its mucin-degrading enzymes. Res Microbiol. 1993; 144:405–410.
Article31. Fenner L, Roux V, Mallet MN, Raoult D. Bacteroides massiliensis sp. nov., isolated from blood culture of a newborn. Int J Syst Evol Microbiol. 2005; 55(Pt 3):1335–1337.
Article32. Ramanan D, Tang MS, Bowcutt R, Loke P, Cadwell K. Bacterial sensor Nod2 prevents inflammation of the small intestine by restricting the expansion of the commensal Bacteroides vulgatus. Immunity. 2014; 41:311–324.
Article33. Mezzatesta ML, Gona F, Stefani S. Enterobacter cloacae complex: clinical impact and emerging antibiotic resistance. Future Microbiol. 2012; 7:887–902.
Article34. Zhu B, Wang S, Li O, et al. High-quality genome sequence of human pathogen Enterobacter asburiae type strain 1497-78T. J Glob Antimicrob Resist. 2017; 8:104–105.
Article35. Chuffa LG, Fioruci-Fontanelli BA, Mendes LO, et al. Melatonin attenuates the TLR4-mediated inflammatory response through MyD88- and TRIF-dependent signaling pathways in an in vivo model of ovarian cancer. BMC Cancer. 2015; 15:34.
Article36. King SJ, McCole DF. Epithelial-microbial diplomacy: escalating border tensions drive inflammation in inflammatory bowel disease. Intest Res. 2019; 17:177–191.
Article37. Zhang YG, Wu S, Xia Y, Sun J. Salmonella infection upregulates the leaky protein claudin-2 in intestinal epithelial cells. PLoS One. 2013; 8:e58606.
Article38. Chelakkot C, Choi Y, Kim DK, et al. Akkermansia muciniphila-derived extracellular vesicles influence gut permeability through the regulation of tight junctions. Exp Mol Med. 2018; 50:e450.
Article39. Ren W, Wang P, Yan J, et al. Melatonin alleviates weanling stress in mice: involvement of intestinal microbiota. J Pineal Res. 2018; 64:e12448.
Article40. Chen L, Wilson JE, Koenigsknecht MJ, et al. NLRP12 attenuates colon inflammation by maintaining colonic microbial diversity and promoting protective commensal bacterial growth. Nat Immunol. 2017; 18:541–551.
Article41. Nishino K, Nishida A, Inoue R, et al. Analysis of endoscopic brush samples identified mucosa-associated dysbiosis in inflammatory bowel disease. J Gastroenterol. 2018; 53:95–106.
Article42. Sartor RB, Wu GD. Roles for intestinal bacteria, viruses, and fungi in pathogenesis of inflammatory bowel diseases and therapeutic approaches. Gastroenterology. 2017; 152:327–339.
Article43. Takahashi K, Nishida A, Fujimoto T, et al. Reduced abundance of butyrate-producing bacteria species in the fecal microbial community in Crohn’s disease. Digestion. 2016; 93:59–65.
Article44. Segata N, Izard J, Waldron L, et al. Metagenomic biomarker discovery and explanation. Genome Biol. 2011; 12:R60.
Article45. Eun CS, Kwak MJ, Han DS, et al. Does the intestinal microbial community of Korean Crohn’s disease patients differ from that of western patients? BMC Gastroenterol. 2016; 16:28.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The effect of tumor resection on intestinal microbiota dysbiosis in patients with right-sided colon cancer
- The Effect of Sleep Disturbances and Melatonin in Inflammatory Bowel Disease
- A Patient with the Disrupted Sleep-Wake Rhythm after Traumatic Brain Injury
- Melatonin Therapy for REM Sleep Behavior Disorder with Co-existing Moderate-to-Severe Sleep Apnea
- Sleep Problems as Predictors in Attention-Deficit Hyperactivity Disorder: Causal Mechanisms, Consequences and Treatment