Cancer Res Treat.
2020 Jul;52(3):747-763. 10.4143/crt.2019.721.
T Cells Modified with CD70 as an Alternative Cellular Vaccine for Antitumor Immunity
- Affiliations
-
- 1Department of Microbiology and Immunology, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 2Translational and Clinical Division, ViGenCell Inc., Seoul, Korea
- 3Catholic Hematopoietic Stem Cell Bank, College of Medicine, The Catholic University of Korea, Seoul, Korea
- KMID: 2504457
- DOI: http://doi.org/10.4143/crt.2019.721
Abstract
- Purpose
Successful tumor eradication primarily depends on generation and maintenance of a large population of tumor-reactive CD8 T cells. Dendritic cells (DCs) are well-known potent antigen-presenting cells and have applied to clinics as potent antitumor therapeutic agents. However, high cost and difficulty in obtaining sufficient amounts for clinical use are the crucial drawbacks of DC-based vaccines. Here, we aimed to develop T cell–based vaccine capable of eliciting potent antitumor therapeutic effects by providing effective costimulatory signals.
Materials and Methods
Antigenic peptide-loaded T cells transfected with retrovirus encoding costimulatory ligands CD70, CD80, OX40L, or 4-1BBL were assessed for antigen-specific CD8 T-cell responses and evaluated antitumor effects along with immunization of a mixture of synthetic peptides, poly-IC and anti-CD40 antibodies (TriVax).
Results
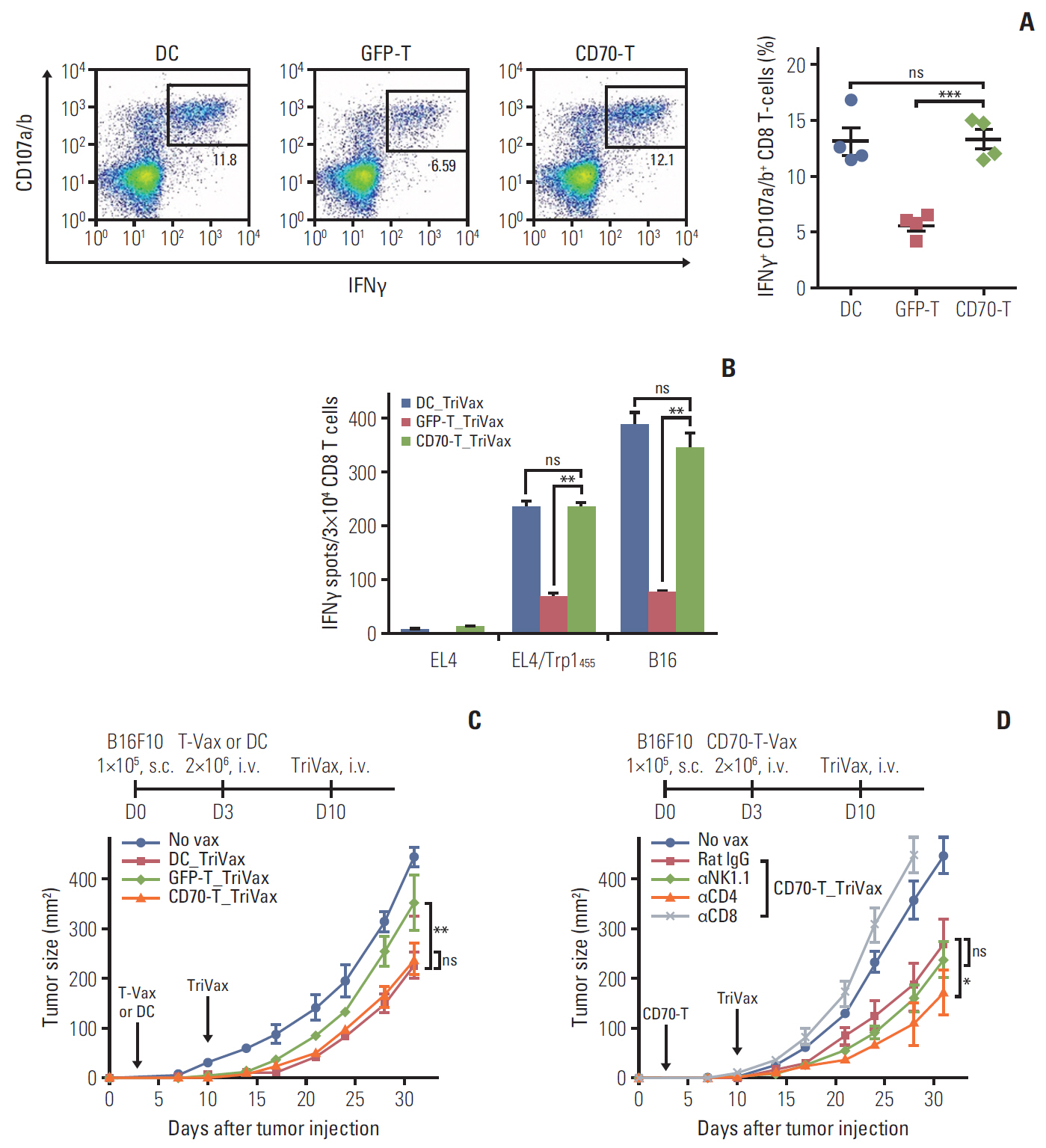
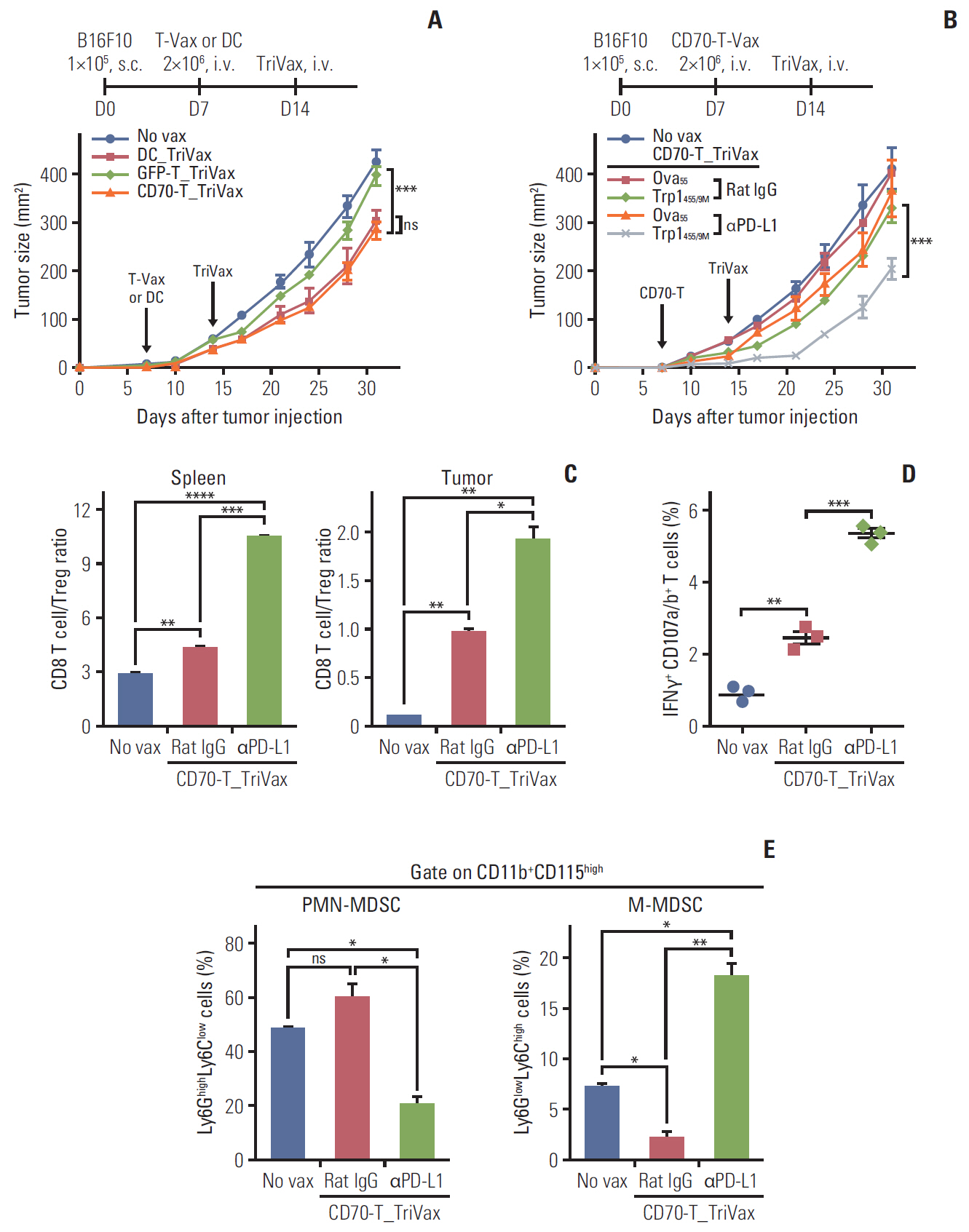
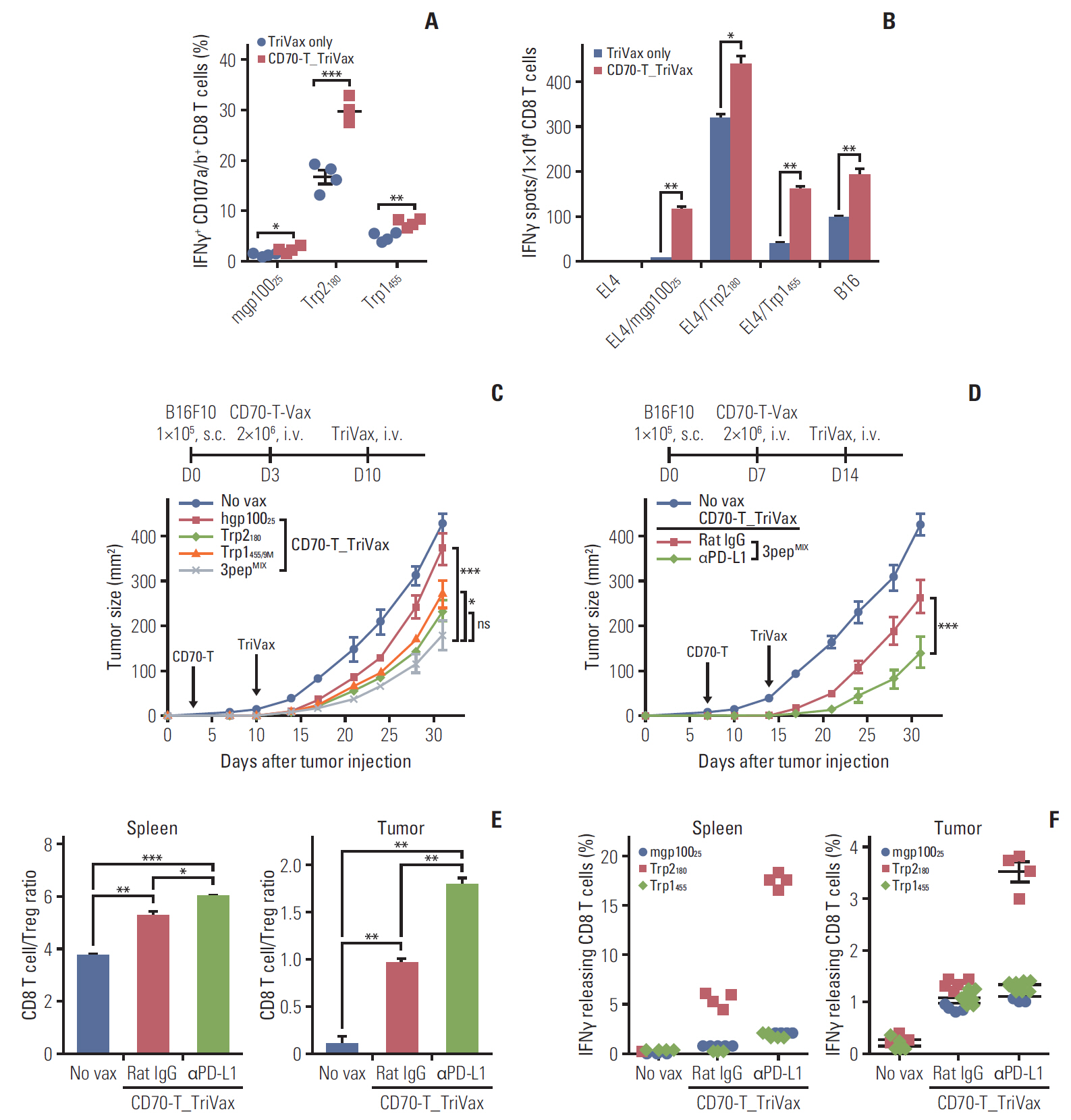
T cells expressing CD70 (CD70-T) exhibited similar level of stimulatory functionality and therapeutic efficacy as DCs. Moreover, CD70-T prime followed by TriVax booster heterologous vaccination elicited therapeutic antitumor effect against B16 melanoma where mediated by CD8 T cells but not CD4 T cells or natural killer cells. The combination with programmed death-ligand 1 blockade led to potent therapeutic efficacy which exhibited increased tumor-infiltrating CD8 T cells. CD70-T pulsed with multi-antigenic peptide generated multiple antigen-specific polyvalent CD8 T cells that were capable of inhibiting tumor growth effectively. Moreover, CD70-T vaccination resulted in higher expansion and migration of adoptively transferred T cells into tumor sites and elicits enhanced therapeutic effects with peptide-based booster immu-nization.
Conclusion
These results imply that T cells endowed with CD70 enable the design of effective vaccination strategies against solid cancer, which may overcome current limitations of DC-based vaccines.
Figure
Reference
-
References
1. Croft M. Co-stimulatory members of the TNFR family: keys to effective T-cell immunity? Nat Rev Immunol. 2003; 3:609–20.
Article2. Thery C, Amigorena S. The cell biology of antigen presentation in dendritic cells. Curr Opin Immunol. 2001; 13:45–51.3. Cho HI, Jung SH, Sohn HJ, Celis E, Kim TG. An optimized peptide vaccine strategy capable of inducing multivalent CD8(+) T cell responses with potent antitumor effects. Oncoimmunology. 2015; 4:e1043504.4. Liu Y, Zhang X, Zhang W, Chen Z, Chan T, Ali K, et al. Adenovirus-mediated CD40 ligand gene-engineered dendritic cells elicit enhanced CD8(+) cytotoxic T-cell activation and antitumor immunity. Cancer Gene Ther. 2002; 9:202–8.
Article5. Mende I, Engleman EG. Breaking tolerance to tumors with dendritic cell-based immunotherapy. Ann N Y Acad Sci. 2005; 1058:96–104.
Article6. Steinman RM, Witmer-Pack M, Inaba K. Dendritic cells: antigen presentation, accessory function and clinical relevance. Adv Exp Med Biol. 1993; 329:1–9.
Article7. Turnis ME, Rooney CM. Enhancement of dendritic cells as vaccines for cancer. Immunotherapy. 2010; 2:847–62.
Article8. Shin CA, Cho HW, Shin AR, Sohn HJ, Cho HI, Kim TG. Co-expression of CD40L with CD70 or OX40L increases B-cell viability and antitumor efficacy. Oncotarget. 2016; 7:46173–86.
Article9. Guo S, Xu J, Denning W, Hel Z. Induction of protective cytotoxic T-cell responses by a B-cell-based cellular vaccine requires stable expression of antigen. Gene Ther. 2009; 16:1300–13.
Article10. Park MY, Kim HS, Woo SJ, Kim CH, Park JS, Sohn HJ, et al. Efficient antitumor immunity in a murine colorectal cancer model induced by CEA RNA-electroporated B cells. Eur J Immunol. 2008; 38:2106–17.
Article11. Kim YJ, Ko HJ, Kim YS, Kim DH, Kang S, Kim JM, et al. alpha-Galactosylceramide-loaded, antigen-expressing B cells prime a wide spectrum of antitumor immunity. Int J Cancer. 2008; 122:2774–83.12. Chung Y, Kim BS, Kim YJ, Ko HJ, Ko SY, Kim DH, et al. CD1d-restricted T cells license B cells to generate long-lasting cytotoxic antitumor immunity in vivo. Cancer Res. 2006; 66:6843–50.
Article13. Himoudi N, Morgenstern DA, Yan M, Vernay B, Saraiva L, Wu Y, et al. Human gammadelta T lymphocytes are licensed for professional antigen presentation by interaction with opsonized target cells. J Immunol. 2012; 188:1708–16.14. Kennedy R, Undale AH, Kieper WC, Block MS, Pease LR, Celis E. Direct cross-priming by th lymphocytes generates memory cytotoxic T cell responses. J Immunol. 2005; 174:3967–77.
Article15. Adamopoulou E, Diekmann J, Tolosa E, Kuntz G, Einsele H, Rammensee HG, et al. Human CD4+ T cells displaying viral epitopes elicit a functional virus-specific memory CD8+ T cell response. J Immunol. 2007; 178:5465–72.
Article16. Park HM, Sohn HJ, Kim YJ, Cho HI, Kim TG. CD4 T-cells transduced with CD80 and 4-1BBL mRNA induce long-term CD8 T-cell responses resulting in potent antitumor effects. Vaccine. 2014; 32:6919–26.
Article17. Croft M. Costimulation of T cells by OX40, 4-1BB, and CD27. Cytokine Growth Factor Rev. 2003; 14:265–73.
Article18. Driessens G, Kline J, Gajewski TF. Costimulatory and coinhibitory receptors in anti-tumor immunity. Immunol Rev. 2009; 229:126–44.
Article19. Johnson BD, Gershan JA, Natalia N, Zujewski H, Weber JJ, Yan X, et al. Neuroblastoma cells transiently transfected to simultaneously express the co-stimulatory molecules CD54, CD80, CD86, and CD137L generate antitumor immunity in mice. J Immunother. 2005; 28:449–60.
Article20. Overwijk WW, Theoret MR, Finkelstein SE, Surman DR, de Jong LA, Vyth-Dreese FA, et al. Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells. J Exp Med. 2003; 198:569–80.
Article21. Garrigan K, Moroni-Rawson P, McMurray C, Hermans I, Abernethy N, Watson J, et al. Functional comparison of spleen dendritic cells and dendritic cells cultured in vitro from bone marrow precursors. Blood. 1996; 88:3508–12.
Article22. Datta J, Terhune JH, Lowenfeld L, Cintolo JA, Xu S, Roses RE, et al. Optimizing dendritic cell-based approaches for cancer immunotherapy. Yale J Biol Med. 2014; 87:491–518.23. Zarnani AH, Torabi-Rahvar M, Bozorgmehr M, Zareie M, Mojtabavi N. Improved efficacy of a dendritic cell-based vaccine against a murine model of colon cancer: the helper protein effect. Cancer Res Treat. 2015; 47:518–26.
Article24. Cho HI, Kim EK, Park SY, Lee SK, Hong YK, Kim TG. Enhanced induction of anti-tumor immunity in human and mouse by dendritic cells pulsed with recombinant TAT fused human survivin protein. Cancer Lett. 2007; 258:189–98.
Article25. Watts TH, Bertram EM, Bukczynski J, Wen T. T cell costimulatory molecules in anti-viral immunity: Potential role in immunotherapeutic vaccines. Can J Infect Dis. 2003; 14:221–9.
Article26. Arens R, Schepers K, Nolte MA, van Oosterwijk MF, van Lier RA, Schumacher TN, et al. Tumor rejection induced by CD70-mediated quantitative and qualitative effects on effector CD8+ T cell formation. J Exp Med. 2004; 199:1595–605.
Article27. Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol. 2007; 8:239–45.
Article28. Duraiswamy J, Kaluza KM, Freeman GJ, Coukos G. Dual blockade of PD-1 and CTLA-4 combined with tumor vaccine effectively restores T-cell rejection function in tumors. Cancer Res. 2013; 73:3591–603.
Article29. Mumprecht S, Schurch C, Schwaller J, Solenthaler M, Ochsenbein AF. Programmed death 1 signaling on chronic myeloid leukemia-specific T cells results in T-cell exhaustion and disease progression. Blood. 2009; 114:1528–36.
Article30. Pilon-Thomas S, Mackay A, Vohra N, Mule JJ. Blockade of programmed death ligand 1 enhances the therapeutic efficacy of combination immunotherapy against melanoma. J Immunol. 2010; 184:3442–9.
Article31. Landsberg J, Kohlmeyer J, Renn M, Bald T, Rogava M, Cron M, et al. Melanomas resist T-cell therapy through inflammation-induced reversible dedifferentiation. Nature. 2012; 490:412–6.
Article32. Mehrotra S, Britten CD, Chin S, Garrett-Mayer E, Cloud CA, Li M, et al. Vaccination with poly(IC:LC) and peptide-pulsed autologous dendritic cells in patients with pancreatic cancer. J Hematol Oncol. 2017; 10:82.
Article33. Aravantinou M, Frank I, Hallor M, Singer R, Tharinger H, Kenney J, et al. PolyICLC exerts pro- and anti-HIV effects on the DC-T cell milieu in vitro and in vivo. PLoS One. 2016; 11:e0161730.
Article34. Johnson P, Challis R, Chowdhury F, Gao Y, Harvey M, Geldart T, et al. Clinical and biological effects of an agonist anti-CD40 antibody: a Cancer Research UK phase I study. Clin Cancer Res. 2015; 21:1321–8.
Article35. Bajor DL, Mick R, Riese MJ, Huang AC, Sullivan B, Richman LP, et al. Long-term outcomes of a phase I study of agonist CD40 antibody and CTLA-4 blockade in patients with metastatic melanoma. Oncoimmunology. 2018; 7:e1468956.
Article36. Kumai T, Fan A, Harabuchi Y, Celis E. Cancer immunotherapy: moving forward with peptide T cell vaccines. Curr Opin Immunol. 2017; 47:57–63.
Article37. Hirayama M, Nishimura Y. The present status and future prospects of peptide-based cancer vaccines. Int Immunol. 2016; 28:319–28.
Article38. Peng M, Mo Y, Wang Y, Wu P, Zhang Y, Xiong F, et al. Neoantigen vaccine: an emerging tumor immunotherapy. Mol Cancer. 2019; 18:128.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- CD70 Expression on Spodoptera Frugiperda(Sf9) Cells by Baculovirus to Induce CD27 Stimulation in Mouse B Cells
- Expression of CD70 during Thymus Regeneration in the Rat
- Influence of Immunity Induced at Priming Step on Mucosal Immunization of Heterologous Prime-Boost Regimens
- Analysis of Antitumor Mechanism of Intravesical BCG Therapy in Tumorigenesis(II) Study about Memory T Cells and Lymphocyte Homing Receptor Positive Cells
- Inflammasomes in antiviral immunity: clues for influenza vaccine development