J Korean Med Sci.
2020 Jul;35(27):e218. 10.3346/jkms.2020.35.e218.
Human Cytomegalovirus (HCMV)-infected Astrocytoma Cells Impair the Function of HCMV-specific Cytotoxic T Cells
- Affiliations
-
- 1Department of Microbiology and Immunology, Seoul National University College of Medicine, Seoul, Korea
- 2BK21 Plus Biomedical Science Project, Seoul National University College of Medicine, Seoul, Korea
- 3Institute of Endemic Diseases, Seoul National University Medical Research Center, Seoul, Korea
- KMID: 2504262
- DOI: http://doi.org/10.3346/jkms.2020.35.e218
Abstract
- Background
Human cytomegalovirus (HCMV) infection in glioblastoma multiforme (GBM) is associated with a poor prognosis and may affect the pathogenesis of GBM. In this study, we investigated the role of HCMV-infected astrocytoma cells in impairing the activity of cytotoxic T lymphocytes (CTLs) specific to the HCMV protein.
Methods
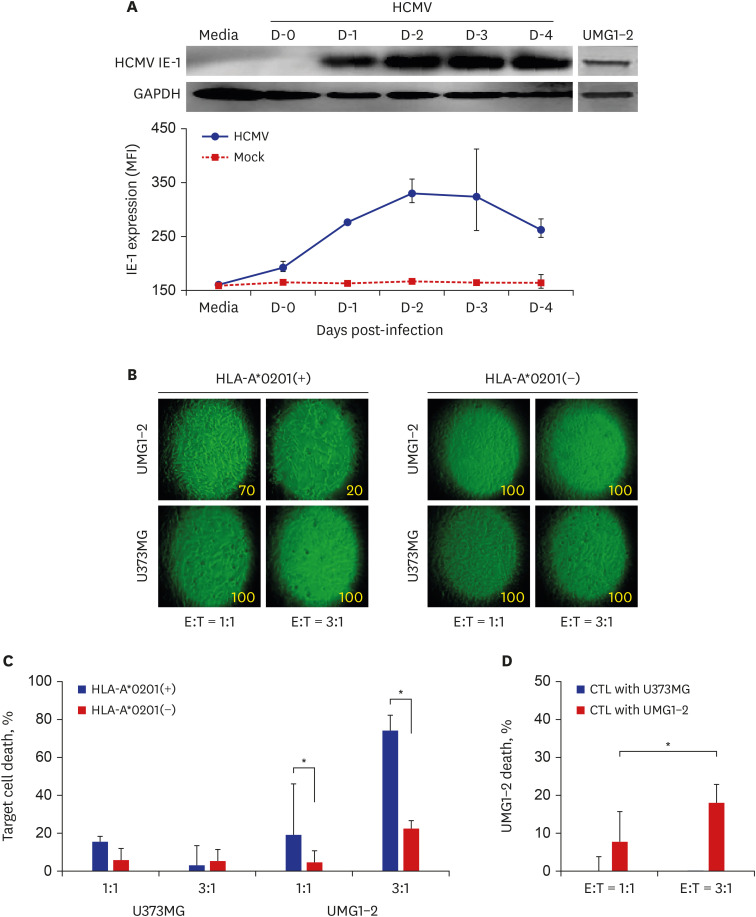
CTLs specific to HCMV immediate early (IE)-1 were expanded from peripheral blood mononuclear cells of healthy donors by stimulating CD8+ T lymphocytes with U373MG cells (ATCC HTB-17: male) expressing HCMV IE-1. The death rate of the target and the effector cells was determined by the total count of the remaining respective cells after the interaction of them.
Results
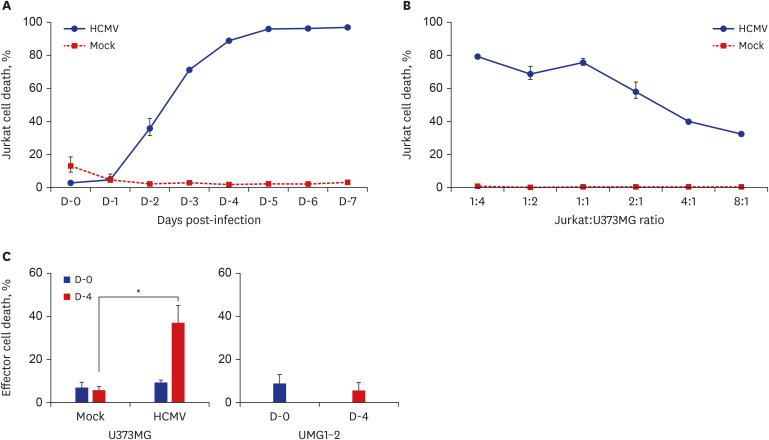
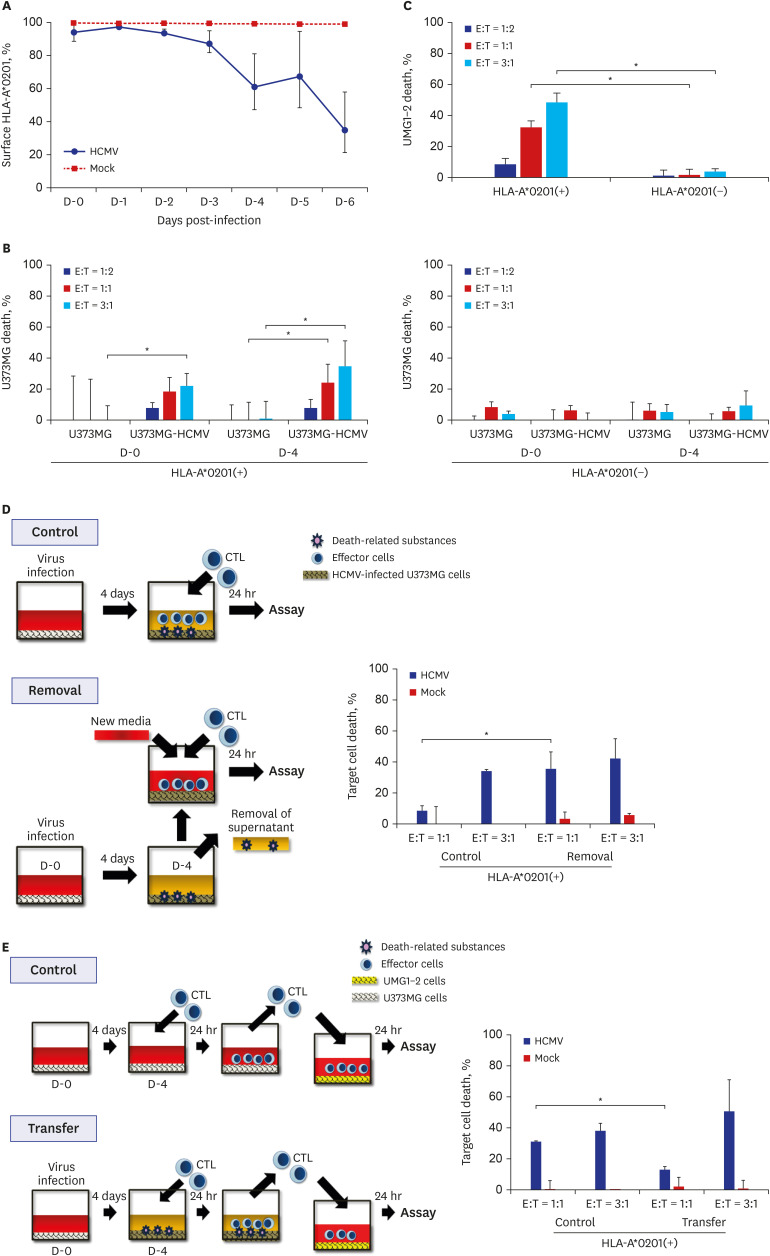
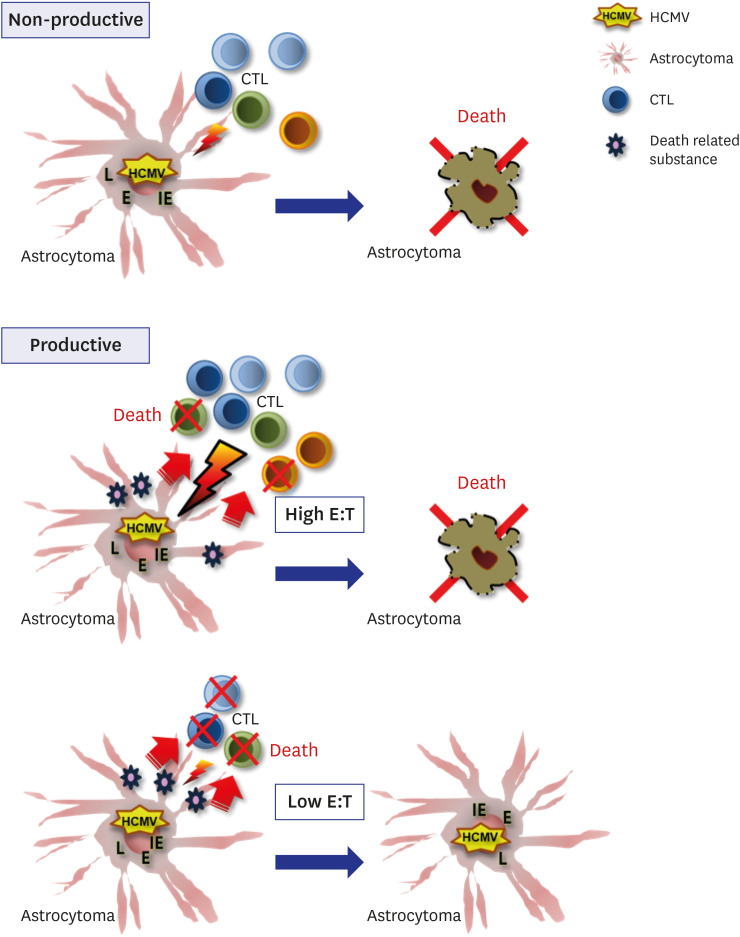
The death rate of the target cells by CTLs increased depending on HLA restriction and the effector:target (E:T) ratio. The death rate of effector cells in the HCMV-infected U373MG cell culture was 37.1% on day 4 post-infection. The removal of the culture supernatant from HCMV-infected U373MG cells prior to adding the effector cells increased target cell death from 8.4% to 40.8% at E:T = 1:1, but not at E:T = 3:1. The transfer of cells from a 24-hour co-culture of the HCMV-infected U373MG cells and CTLs to HCMV IE-1-expressing target cells resulted in decreasing the cell death rate of the target cells from 31.1% to 13.0% at E:T = 1:1, but not at E:T = 3:1. HCMV infection of U373MG cells decreases the activity of CTLs specific to HCMV when the number of CTLs is low.
Conclusion
These results suggest that HCMV could impair CTL activity and facilitate glioblastoma growth unchecked by CTLs.
Keyword
Figure
Reference
-
1. Dolecek TA, Propp JM, Stroup NE, Kruchko C. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2005–2009. Neuro-oncol. 2012; 14 Suppl 5:v1–v49. PMID: 23095881.
Article2. Garside R, Pitt M, Anderson R, Rogers G, Dyer M, Mealing S, et al. The effectiveness and cost-effectiveness of carmustine implants and temozolomide for the treatment of newly diagnosed high-grade glioma: a systematic review and economic evaluation. Health Technol Assess. 2007; 11(45):iii–iv.
Article3. Tang KW, Hellstrand K, Larsson E. Absence of cytomegalovirus in high-coverage DNA sequencing of human glioblastoma multiforme. Int J Cancer. 2015; 136(4):977–981. PMID: 24961996.
Article4. Baumgarten P, Michaelis M, Rothweiler F, Starzetz T, Rabenau HF, Berger A, et al. Human cytomegalovirus infection in tumor cells of the nervous system is not detectable with standardized pathologico-virological diagnostics. Neuro-oncol. 2014; 16(11):1469–1477. PMID: 25155358.
Article5. Mitchell DA, Xie W, Schmittling R, Learn C, Friedman A, McLendon RE, et al. Sensitive detection of human cytomegalovirus in tumors and peripheral blood of patients diagnosed with glioblastoma. Neuro-oncol. 2008; 10(1):10–18. PMID: 17951512.
Article6. Prins RM, Cloughesy TF, Liau LM. Cytomegalovirus immunity after vaccination with autologous glioblastoma lysate. N Engl J Med. 2008; 359(5):539–541. PMID: 18669440.
Article7. Hong S, Van Kaer L. Immune privilege: keeping an eye on natural killer T cells. J Exp Med. 1999; 190(9):1197–1200. PMID: 10544192.8. Mitchell DA, Fecci PE, Sampson JH. Immunotherapy of malignant brain tumors. Immunol Rev. 2008; 222(1):70–100. PMID: 18363995.
Article9. Wekerle H, Sun D, Oropeza-Wekerle RL, Meyermann R. Immune reactivity in the nervous system: modulation of T-lymphocyte activation by glial cells. J Exp Biol. 1987; 132:43–57. PMID: 3323405.
Article10. Hertel L, Chou S, Mocarski ES. Viral and cell cycle-regulated kinases in cytomegalovirus-induced pseudomitosis and replication. PLoS Pathog. 2007; 3(1):e6. PMID: 17206862.
Article11. Jackson SE, Mason GM, Wills MR. Human cytomegalovirus immunity and immune evasion. Virus Res. 2011; 157(2):151–160. PMID: 21056604.
Article12. Hadrup SR, Strindhall J, Køllgaard T, Seremet T, Johansson B, Pawelec G, et al. Longitudinal studies of clonally expanded CD8 T cells reveal a repertoire shrinkage predicting mortality and an increased number of dysfunctional cytomegalovirus-specific T cells in the very elderly. J Immunol. 2006; 176(4):2645–2653. PMID: 16456027.
Article13. Slavuljica I, Kveštak D, Huszthy PC, Kosmac K, Britt WJ, Jonjić S. Immunobiology of congenital cytomegalovirus infection of the central nervous system—the murine cytomegalovirus model. Cell Mol Immunol. 2015; 12(2):180–191. PMID: 25042632.
Article14. Han S, Zhang C, Li Q, Dong J, Liu Y, Huang Y, et al. Tumour-infiltrating CD4(+) and CD8(+) lymphocytes as predictors of clinical outcome in glioma. Br J Cancer. 2014; 110(10):2560–2568. PMID: 24691423.
Article15. Kim JH, Kim J, Roh J, Park CS, Seoh JY, Hwang ES. Reactive oxygen species-induced parthanatos of immunocytes by human cytomegalovirus-associated substance. Microbiol Immunol. 2018; 62(4):229–242. PMID: 29350405.
Article16. Kim J, Kwon YJ, Park ES, Sung B, Kim JH, Park CG, et al. Human cytomegalovirus (HCMV) IE1 plays role in resistance to apoptosis with etoposide in cancer cell line by Cdk2 accumulation. Microbiol Immunol. 2003; 47(12):959–967. PMID: 14695446.
Article17. Shin KC, Park CG, Hwang ES, Cha CY. Human cytomegalovirus IE1 protein enhances herpes simplex virus type 1-induced syncytial formation in U373MG cells. J Korean Med Sci. 2008; 23(6):1046–1052. PMID: 19119451.
Article18. Kern F, Surel IP, Faulhaber N, Frömmel C, Schneider-Mergener J, Schönemann C, et al. Target structures of the CD8(+)-T-cell response to human cytomegalovirus: the 72-kilodalton major immediate-early protein revisited. J Virol. 1999; 73(10):8179–8184. PMID: 10482568.
Article19. McLaughlin-Taylor E, Pande H, Forman SJ, Tanamachi B, Li CR, Zaia JA, et al. Identification of the major late human cytomegalovirus matrix protein pp65 as a target antigen for CD8+ virus-specific cytotoxic T lymphocytes. J Med Virol. 1994; 43(1):103–110. PMID: 8083644.
Article20. Slezak SL, Bettinotti M, Selleri S, Adams S, Marincola FM, Stroncek DF. CMV pp65 and IE-1 T cell epitopes recognized by healthy subjects. J Transl Med. 2007; 5(1):17. PMID: 17391521.
Article21. Scheurer ME, Bondy ML, Aldape KD, Albrecht T, El-Zein R. Detection of human cytomegalovirus in different histological types of gliomas. Acta Neuropathol. 2008; 116(1):79–86. PMID: 18351367.
Article22. Lim JB, Kim HO, Jeong SH, Ha JE, Jang S, Lee SG, et al. Identification of HLA-A*2402-restricted HCMV immediate early-1 (IE-1) epitopes as targets for CD8+ HCMV-specific cytotoxic T lymphocytes. J Transl Med. 2009; 7(1):72. PMID: 19698161.
Article23. Michelson S. Human cytomegalovirus escape from immune detection. Intervirology. 1999; 42(5-6):301–307. PMID: 10702710.
Article24. Aptsiauri N, Cabrera T, Mendez R, Garcia-Lora A, Ruiz-Cabello F, Garrido F. Role of altered expression of HLA class I molecules in cancer progression. Adv Exp Med Biol. 2007; 601:123–131. PMID: 17712999.
Article25. Goepel JR, Rees RC, Rogers K, Stoddard CJ, Thomas WE, Shepherd L. Loss of monomorphic and polymorphic HLA antigens in metastatic breast and colon carcinoma. Br J Cancer. 1991; 64(5):880–883. PMID: 1718386.
Article26. Fletcher JM, Prentice HG, Grundy JE. Natural killer cell lysis of cytomegalovirus (CMV)-infected cells correlates with virally induced changes in cell surface lymphocyte function-associated antigen-3 (LFA-3) expression and not with the CMV-induced down-regulation of cell surface class I HLA. J Immunol. 1998; 161(5):2365–2374. PMID: 9725232.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Eukaryotic Expression of Human Cytomegalovirus ( HCMV ) Glycoprotein H ( gH )
- Effect of Human Cytomegalovirus on Human Monocyte-derived Dendritic Cells
- Effect of Human Cytomegalovirus ( HCMV ) Replication on the Production of Alkaline Phosphatase in Osteosarcoma Cell Line ( Saos - 2 )
- Cytoplasmic Translocation of p53 by Human Cytomegalovirus Infection
- Little role of anti-gB antibodies in neutralizing activity of patient's sera with human cytomegalovirus (HCMV) infection