Anat Cell Biol.
2020 Jun;53(2):169-182. 10.5115/acb.19.231.
Effect of meloxicam (cyclooygenase-2 inhibitor) versus vitamin D3 (cholecalciferol) as ameliorating agents of progressive doxorubicin-induced nephrotoxicity in rats
- Affiliations
-
- 1Department of Anatomy, Faculty of Medicine, Mansoura University, Mansoura, Egypt
- 2Department of Clinical Laboratory Sciences, College of Applied Medical Sciences, Jouf University, Sakaka, Saudi Arabia
- 3Department of Basic Medical Sciences, College of Medicine, Princess Nourah Bint Abdulrahman University, Riyadh, Saudi Arabia
- KMID: 2503447
- DOI: http://doi.org/10.5115/acb.19.231
Abstract
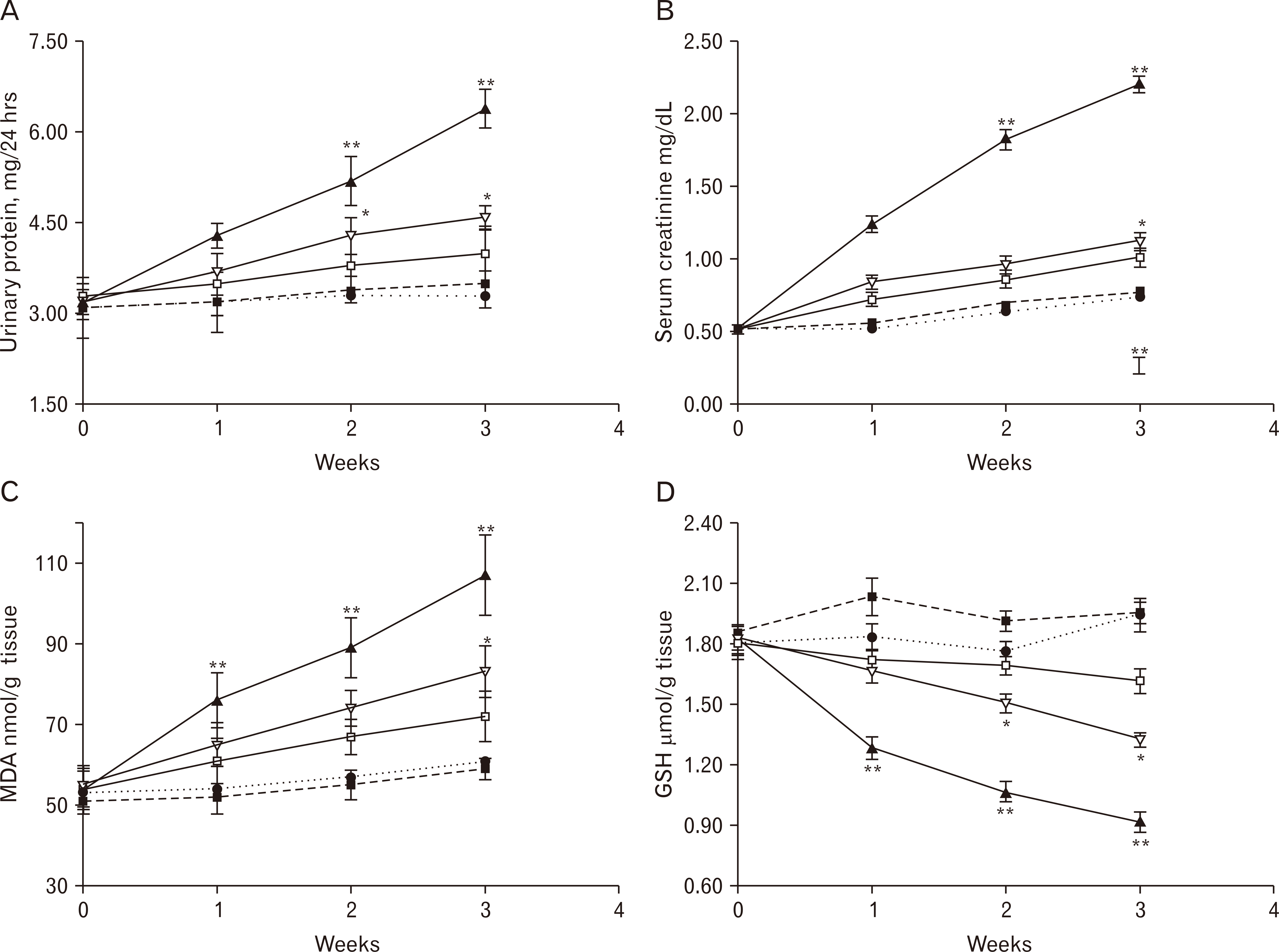
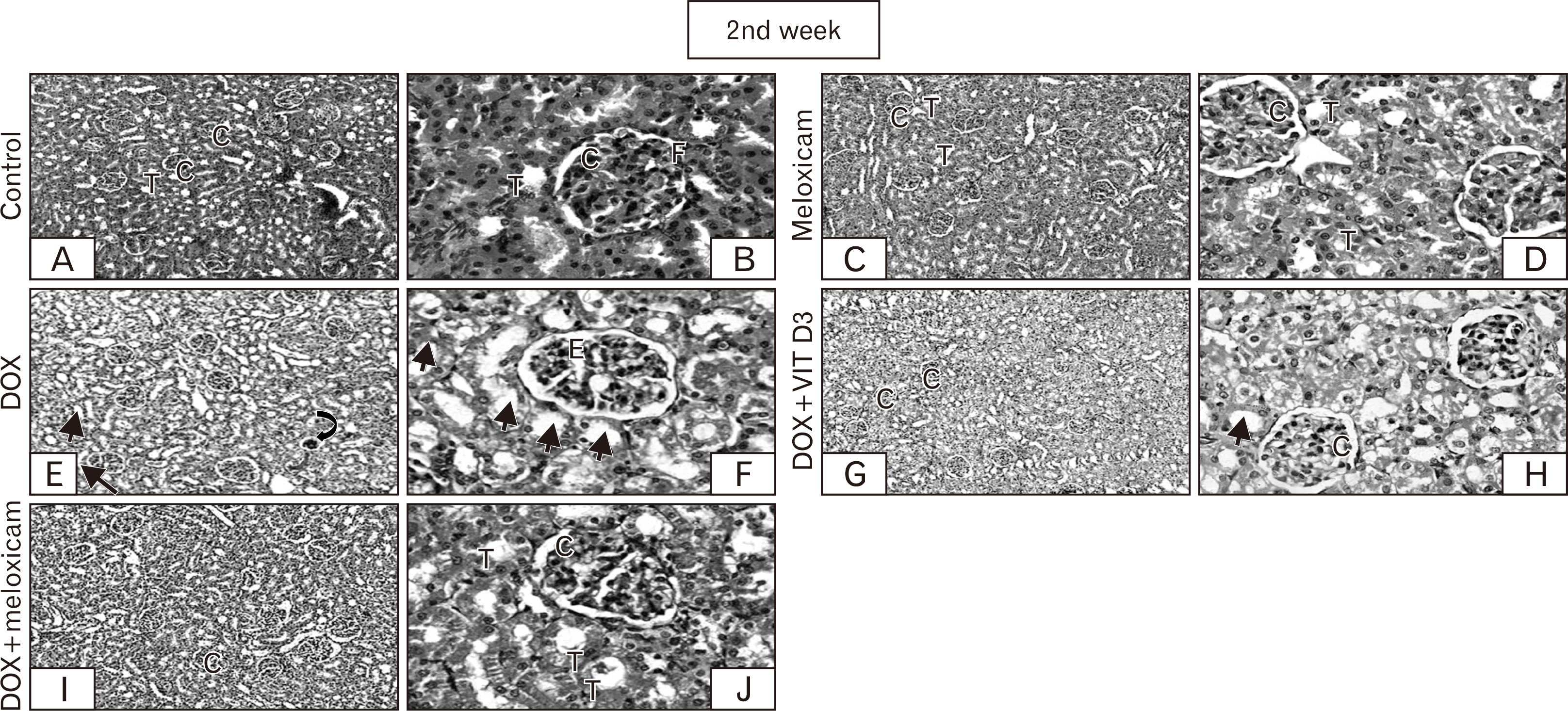
- Doxorubicin (DOX)-induced nephropathy hampered its antineoplastic efficiency. The objective of the current work is to assess the prospective ameliorating effects of meloxicam versus vitamin D3 (Vit D3, cholecalciferol) against progressive DOX-induced nephropathy in rats trying to ascertain the possible mechanism underlying such amelioration. Ninety Male Wistar rats were randomly distributed to five experimental groups for 3 weeks, with saline, meloxicam (daily), DOX (single dose), Vit D3+DOX, or both meloxicam and DOX. We measured levels of urinary protein, serum creatinine, malondialdehyde (MDA) and renal reduced glutathione (GSH). In addition, tumor necrosis factor-alpha (TNF-α) expression and renal histopathology were assessed. Meloxicam alone treated group revealed no significant difference in urinary protein and serum creatinine. It also presented non-significant reduction in the MDA content while an increase in the reduced GSH content in contrast to the control group, which is more evident after the first week. Renal sections of rats received meloxicam only showed no significant histological changes and negative immunoreactivity compared to the control group. DOX induced a significant increase in urinary protein, serum creatinine, decrease reduced GSH, increased renal MDA and disrupted renal morphometric parameters and histology with increased TNF-α expression. Combination groups of Vit D3+DOX and meloxicam+DOX showed improvement of all DOX disturbed parameters. Meloxicam showed better results most likely due to anti-inflammatory and antioxidant activities superimposing the immune-modulatory effect of Vit D3. So, it is recommended to use meloxicam in patients receiving DOX as a renoprotective agent in addition to its analgesic effects required by cancer patients.
Keyword
Figure
Reference
-
1. El-Sheikh AA, Morsy MA, Mahmoud MM, Rifaai RA, Abdelrahman AM. 2012; Effect of coenzyme-q10 on Doxorubicin-induced nephrotoxicity in rats. Adv Pharmacol Sci. 2012:981461. DOI: 10.1155/2012/981461. PMID: 23346106. PMCID: PMC3533995.
Article2. Ramadan R, Faour D, Awad H, Khateeb E, Cohen R, Yahia A, Torgovicky R, Cohen R, Lazari D, Kawachi H, Abassi Z. 2012; Early treatment with everolimus exerts nephroprotective effect in rats with adriamycin-induced nephrotic syndrome. Nephrol Dial Transplant. 27:2231–41. DOI: 10.1093/ndt/gfr581. PMID: 22036940.
Article3. Wang JL, Cheng HF, Shappell S, Harris RC. 2000; A selective cyclooxygenase-2 inhibitor decreases proteinuria and retards progressive renal injury in rats. Kidney Int. 57:2334–42. DOI: 10.1046/j.1523-1755.2000.00093.x. PMID: 10844603.
Article4. Dabak DO, Kuloglu T, Ozercan MR. 2009; Effects of vitamin D3 (cholecalciferol) on adriamycin-induced nephrotoxicity. Ren Fail. 31:400–5. DOI: 10.1080/08860220902883020. PMID: 19839841.5. Di Rosa M, Malaguarnera M, Nicoletti F, Malaguarnera L. 2011; Vitamin D3: a helpful immuno-modulator. Immunology. 134:123–39. DOI: 10.1111/j.1365-2567.2011.03482.x. PMID: 21896008. PMCID: PMC3194221.
Article6. Szymczak I, Pawliczak R. 2016; The active metabolite of vitamin D3 as a potential immunomodulator. Scand J Immunol. 83:83–91. DOI: 10.1111/sji.12403. PMID: 26678915.7. Hewison M. 2012; Vitamin D and the immune system: new perspectives on an old theme. Rheum Dis Clin North Am. 38:125–39. DOI: 10.1016/j.rdc.2012.03.012. PMID: 22525848.
Article8. Baek S, Lee YS, Shim HE, Yoon S, Baek SY, Kim BS, Oh SO. 2011; Vitamin D3 regulates cell viability in gastric cancer and cholangiocarcinoma. Anat Cell Biol. 44:204–9. DOI: 10.5115/acb.2011.44.3.204. PMID: 22025972. PMCID: PMC3195824.
Article9. Zou MS, Yu J, Zhou JH, Nie GM, Ding DS, Luo LM, Xu HT, He WS. 2010; 1,25-Dihydroxyvitamin D3 ameliorates podocytopenia in rats with adriamycin-induced nephropathy. Intern Med. 49:2677–86. DOI: 10.2169/internalmedicine.49.4174. PMID: 21173542.
Article10. Deepa PR, Varalakshmi P. 2003; The cytoprotective role of a low-molecular-weight heparin fragment studied in an experimental model of glomerulotoxicity. Eur J Pharmacol. 478:199–205. DOI: 10.1016/j.ejphar.2003.08.084. PMID: 14575805.
Article11. Hassan MH, Ghobara M, Abd-Allah GM. 2014; Modulator effects of meloxicam against doxorubicin-induced nephrotoxicity in mice. J Biochem Mol Toxicol. 28:337–46. DOI: 10.1002/jbt.21570. PMID: 24799355.
Article12. Puhlmann U, Ziemann C, Ruedell G, Vorwerk H, Schaefer D, Langebrake C, Schuermann P, Creutzig U, Reinhardt D. 2005; Impact of the cyclooxygenase system on doxorubicin-induced functional multidrug resistance 1 overexpression and doxorubicin sensitivity in acute myeloid leukemic HL-60 cells. J Pharmacol Exp Ther. 312:346–54. DOI: 10.1124/jpet.104.071571. PMID: 15501994.
Article13. Han HK, Choi HK. 2007; Improved absorption of meloxicam via salt formation with ethanolamines. Eur J Pharm Biopharm. 65:99–103. DOI: 10.1016/j.ejpb.2006.07.003. PMID: 16919925.
Article14. Edfawy M, Hassan MH, Mansour A, Hamed AA, Amin HA. 2012; Meloxicam modulates oxidative stress status, inhibits prostaglandin E2, and abrogates apoptosis in carbon tetrachloride-induced rat hepatic injury. Int J Toxicol. 31:276–86. DOI: 10.1177/1091581812442939. PMID: 22556387.
Article15. Refaie MM, Amin EF, El-Tahawy NF, Abdelrahman AM. 2016; Possible protective effect of diacerein on doxorubicin-induced nephrotoxicity in rats. J Toxicol. 2016:9507563. DOI: 10.1155/2016/9507563. PMID: 26904117. PMCID: PMC4745331.
Article16. Moron MS, Depierre JW, Mannervik B. 1979; Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim Biophys Acta. 582:67–78. DOI: 10.1016/0304-4165(79)90289-7. PMID: 760819.
Article17. Mihara M, Uchiyama M. 1983; Properties of thiobarbituric acid-reactive materials obtained from lipid peroxide and tissue homogenate. Chem Pharm Bull (Tokyo). 31:605–11. DOI: 10.1248/cpb.31.605. PMID: 6883588.
Article18. Nestor SL, Bancroft JD. Bancroft JD, Stevens A, editors. 1996. Enzyme histochemistry and its diagnostic applications. Theory and practice of histological techniques. Churchill Livingstone;New York:
Article19. Côté S, Ribeiro-da-Silva A, Cuello AC. Cuello AC, editor. 1993. Current protocols for light microscopy immunohistochemistry. Immunohistochemistry II. Wiley;Chichester: p. 148–67.20. Sanden SK, Wiggins JE, Goyal M, Riggs LK, Wiggins RC. 2003; Oct. Evaluation of a thick and thin section method for estimation of podocyte number, glomerular volume, and glomerular volume per podocyte in rat kidney with Wilms' tumor-1 protein used as a podocyte nuclear marker. J Am Soc Nephrol. 14:2484–93. DOI: 10.1097/01.ASN.0000089829.45296.7C. PMID: 14514726.
Article21. Véniant M, Heudes D, Clozel JP, Bruneval P, Ménard J. 1994; Calcium blockade versus ACE inhibition in clipped and unclipped kidneys of 2K-1C rats. Kidney Int. 46:421–9. DOI: 10.1038/ki.1994.290. PMID: 7967354.
Article22. el Nahas AM, Bassett AH, Cope GH, Le Carpentier JE. 1991; Role of growth hormone in the development of experimental renal scarring. Kidney Int. 40:29–34. DOI: 10.1038/ki.1991.175. PMID: 1921152.
Article23. Fedchenko N, Reifenrath J. 2014; Different approaches for interpretation and reporting of immunohistochemistry analysis results in the bone tissue - a review. Diagn Pathol. 9:221. DOI: 10.1186/s13000-014-0221-9. PMID: 25432701. PMCID: PMC4260254.
Article24. Bankhead P, Loughrey MB, Fernández JA, Dombrowski Y, McArt DG, Dunne PD, McQuaid S, Gray RT, Murray LJ, Coleman HG, James JA, Salto-Tellez M, Hamilton PW. 2017; QuPath: open source software for digital pathology image analysis. Sci Rep. 7:16878. DOI: 10.1038/s41598-017-17204-5. PMID: 29203879. PMCID: PMC5715110.
Article25. Rayson D, Richel D, Chia S, Jackisch C, van der Vegt S, Suter T. 2008; Anthracycline-trastuzumab regimens for HER2/neu-overexpressing breast cancer: current experience and future strategies. Ann Oncol. 19:1530–9. DOI: 10.1093/annonc/mdn292. PMID: 18480068.
Article26. Taskin E, Dursun N. 2012; The protection of selenium on adriamycin-induced mitochondrial damage in rat. Biol Trace Elem Res. 147:165–71. DOI: 10.1007/s12011-011-9273-9. PMID: 22237420.
Article27. Kadiiska MB, Gladen BC, Baird DD, Germolec D, Graham LB, Parker CE, Nyska A, Wachsman JT, Ames BN, Basu S, Brot N, Fitzgerald GA, Floyd RA, George M, Heinecke JW, Hatch GE, Hensley K, Lawson JA, Marnett LJ, Morrow JD, Murray DM, Plastaras J, Roberts LJ 2nd, Rokach J, Shigenaga MK, Sohal RS, Sun J, Tice RR, Van Thiel DH, Wellner D, Walter PB, Tomer KB, Mason RP, Barrett JC. 2005; Biomarkers of oxidative stress study II: are oxidation products of lipids, proteins, and DNA markers of CCl4 poisoning? Free Radic Biol Med. 38:698–710. DOI: 10.1016/j.freeradbiomed.2004.09.017. PMID: 15721980.
Article28. Oguz F, Beytur A, Sarihan E, Oguz HK, Bentli R, Samdanci E, Kose E, Polat A, Duran ZR, Parlakpinar H, Ekinci N. 2016; Protective effects of molsidomine against doxorubicin-induced renal damage in rats. Clin Invest Med. 39:E7–14. DOI: 10.25011/cim.v39i1.26325. PMID: 26833172.
Article29. Federico A, Morgillo F, Tuccillo C, Ciardiello F, Loguercio C. 2007; Chronic inflammation and oxidative stress in human carcinogenesis. Int J Cancer. 121:2381–6. DOI: 10.1002/ijc.23192. PMID: 17893868.
Article30. Rios A, Vargas-Robles H, Gámez-Méndez AM, Escalante B. 2012; Cyclooxygenase-2 and kidney failure. Prostaglandins Other Lipid Mediat. 98:86–90. DOI: 10.1016/j.prostaglandins.2011.11.004. PMID: 22119250.
Article31. Tomiyama-Hanayama M, Rakugi H, Kohara M, Mima T, Adachi Y, Ohishi M, Katsuya T, Hoshida Y, Aozasa K, Ogihara T, Nishimoto N. 2009; Effect of interleukin-6 receptor blockage on renal injury in apolipoprotein E-deficient mice. Am J Physiol Renal Physiol. 297:F679–84. DOI: 10.1152/ajprenal.90680.2008. PMID: 19570877.
Article32. Yagmurca M, Erdogan H, Iraz M, Songur A, Ucar M, Fadillioglu E. 2004; Caffeic acid phenethyl ester as a protective agent against doxorubicin nephrotoxicity in rats. Clin Chim Acta. 348:27–34. DOI: 10.1016/j.cccn.2004.03.035. PMID: 15369732.
Article33. Ajith TA, Aswathy MS, Hema U. 2008; Protective effect of Zingiber officinale roscoe against anticancer drug doxorubicin-induced acute nephrotoxicity. Food Chem Toxicol. 46:3178–81. DOI: 10.1016/j.fct.2008.07.004. PMID: 18680783.
Article34. Mohan M, Kamble S, Gadhi P, Kasture S. 2010; Protective effect of Solanum torvum on doxorubicin-induced nephrotoxicity in rats. Food Chem Toxicol. 48:436–40. DOI: 10.1016/j.fct.2009.10.042. PMID: 19883716.
Article35. Komers R, Lindsley JN, Oyama TT, Anderson S. 2007; Cyclo-oxygenase-2 inhibition attenuates the progression of nephropathy in uninephrectomized diabetic rats. Clin Exp Pharmacol Physiol. 34:36–41. DOI: 10.1111/j.1440-1681.2007.04534.x. PMID: 17201733.
Article36. Xu S, Chen YH, Tan ZX, Xie DD, Zhang C, Zhang ZH, Wang H, Zhao H, Yu DX, Xu DX. 2015; Vitamin D3 pretreatment regulates renal inflammatory responses during lipopolysaccharide-induced acute kidney injury. Sci Rep. 5:18687. DOI: 10.1038/srep18687. PMID: 26691774. PMCID: PMC4686931.
Article37. Javaid B, Olson JL, Meyer TW. 2001; Glomerular injury and tubular loss in Adriamycin nephrosis. J Am Soc Nephrol. 12:1391–400. PMID: 11423568.
Article38. Mansouri E, Assarehzadegan MA, Nejad-Dehbashi F, Kooti W. 2018; Effects of pravastatin in adriamycin-induced nephropathy in rats. Iran J Pharm Res. 17:1413–9. PMID: 30568699. PMCID: PMC6269566.39. Remuzzi G, Bertani T. 1998; Pathophysiology of progressive nephropathies. N Engl J Med. 339:1448–56. DOI: 10.1056/NEJM199811123392007. PMID: 9811921.
Article40. Rashid S, Ali N, Nafees S, Ahmad ST, Arjumand W, Hasan SK, Sultana S. 2013; Alleviation of doxorubicin-induced nephrotoxicity and hepatotoxicity by chrysin in Wistar rats. Toxicol Mech Methods. 23:337–45. DOI: 10.3109/15376516.2012.759306. PMID: 23256457.
Article41. Al-Saedi HF, Al-Zubaidy AA, Khattab YI. 2014; The possible effects of montelukast against doxorubicin-induced nephrotoxicity in rabbits. Int J Adv Res. 2:723–9.42. Tian J, Liu Y, Williams LA, de Zeeuw D. 2007; Potential role of active vitamin D in retarding the progression of chronic kidney disease. Nephrol Dial Transplant. 22:321–8. DOI: 10.1093/ndt/gfl595. PMID: 17121787.
Article43. He W, Kang YS, Dai C, Liu Y. 2011; Blockade of Wnt/b-catenin signaling by paricalcitol ameliorates proteinuria and kidney injury. J Am Soc Nephrol. 22:90–103. DOI: 10.1681/ASN.2009121236. PMID: 21030600. PMCID: PMC3014038.44. Panichi V, Migliori M, Taccola D, Filippi C, De Nisco L, Giovannini L, Palla R, Tetta C, Camussi G. 2001; Effects of 1,25(OH)2D3 in experimental mesangial proliferative nephritis in rats. Kidney Int. 60:87–95. DOI: 10.1046/j.1523-1755.2001.00775.x. PMID: 11422740.
Article45. Chien SC, Wu YC, Chen ZW, Yang WC. 2015; Naturally occurring anthraquinones: chemistry and therapeutic potential in autoimmune diabetes. Evid Based Complement Alternat Med. 2015:357357. DOI: 10.1155/2015/357357. PMID: 25866536. PMCID: PMC4381678.
Article46. Qiu S, Sun G, Zhang Y, Li X, Wang R. 2016; Involvement of the NF-κB signaling pathway in the renoprotective effects of isorhamnetin in a type 2 diabetic rat model. Biomed Rep. 4:628–34. DOI: 10.3892/br.2016.636. PMID: 27123259. PMCID: PMC4840710.
Article47. Sanchez PL, Salgado LM, Ferreri NR, Escalante B. 1999; Effect of cyclooxygenase-2 inhibition on renal function after renal ablation. Hypertension. 34(4 Pt 2):848–53. DOI: 10.1161/01.HYP.34.4.848. PMID: 10523372.
Article48. Wang Y, Wang YP, Tay YC, Harris DC. 2000; Progressive adriamycin nephropathy in mice: sequence of histologic and immunohistochemical events. Kidney Int. 58:1797–804. DOI: 10.1046/j.1523-1755.2000.00342.x. PMID: 11012915.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Vitamin D3 regulates cell viability in gastric cancer and cholangiocarcinoma
- The Effect of 1, 25-Dihydroxyvitamin D3 on Dopaminergic Neurons and Microglial Activation in Parkinsonian Rat Model Induced by 6-Hydroxydopamine
- Efficacy of plain cholecalciferol versus ergocalciferol in raising serum vitamin D level in Thai female healthcare workers
- The Role of The Effects of Vitamin D Supplementation on Bone Mineral Density and Bone Mineral Content in Ovariectomized Rats Compensation in Rats
- Exploring optimal supplementation for people with vitamin D deficiency