Ann Lab Med.
2020 Sep;40(5):398-408. 10.3343/alm.2020.40.5.398.
Pre-Transplant Angiotensin II Type 1 Receptor Antibodies and Anti-Endothelial Cell Antibodies Predict Graft Function and Allograft Rejection in a Low-Risk Kidney Transplantation Setting
- Affiliations
-
- 1Department of Laboratory Medicine, Haeundae Paik Hospital, Inje University College of Medicine, Busan, Korea
- 2Departments of Laboratory Medicine and Genetics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 3Departments of Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 4Departments of Organ Transplantation Center, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 5Departments of Internal Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 6Departments of Pathology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 7Department of Surgery Kosin University Gospel Hospital, Medical College of Kosin University, Busan, Korea
- KMID: 2502570
- DOI: http://doi.org/10.3343/alm.2020.40.5.398
Abstract
- Background
Non-HLA antibodies, anti-angiotensin II type 1 receptor antibodies (anti-AT1R) and anti-endothelial cell antibodies (AECA), are known to play a role in allograft rejection. We evaluated the role of both antibodies in predicting post-transplant outcomes in low-risk living donor kidney transplantation (LDKT) recipients.
Methods
In 94 consecutive LDKT recipients who were ABO compatible and negative for pre-transplant HLA donor-specific antibodies, we determined the levels of anti-AT1Rs using an enzyme-linked immunosorbent assay and the presence of AECAs using a flow cytometric endothelial cell crossmatch (ECXM) assay with pre-transplant sera. Hazard ratio (HR) was calculated to predict post-transplant outcomes.
Results
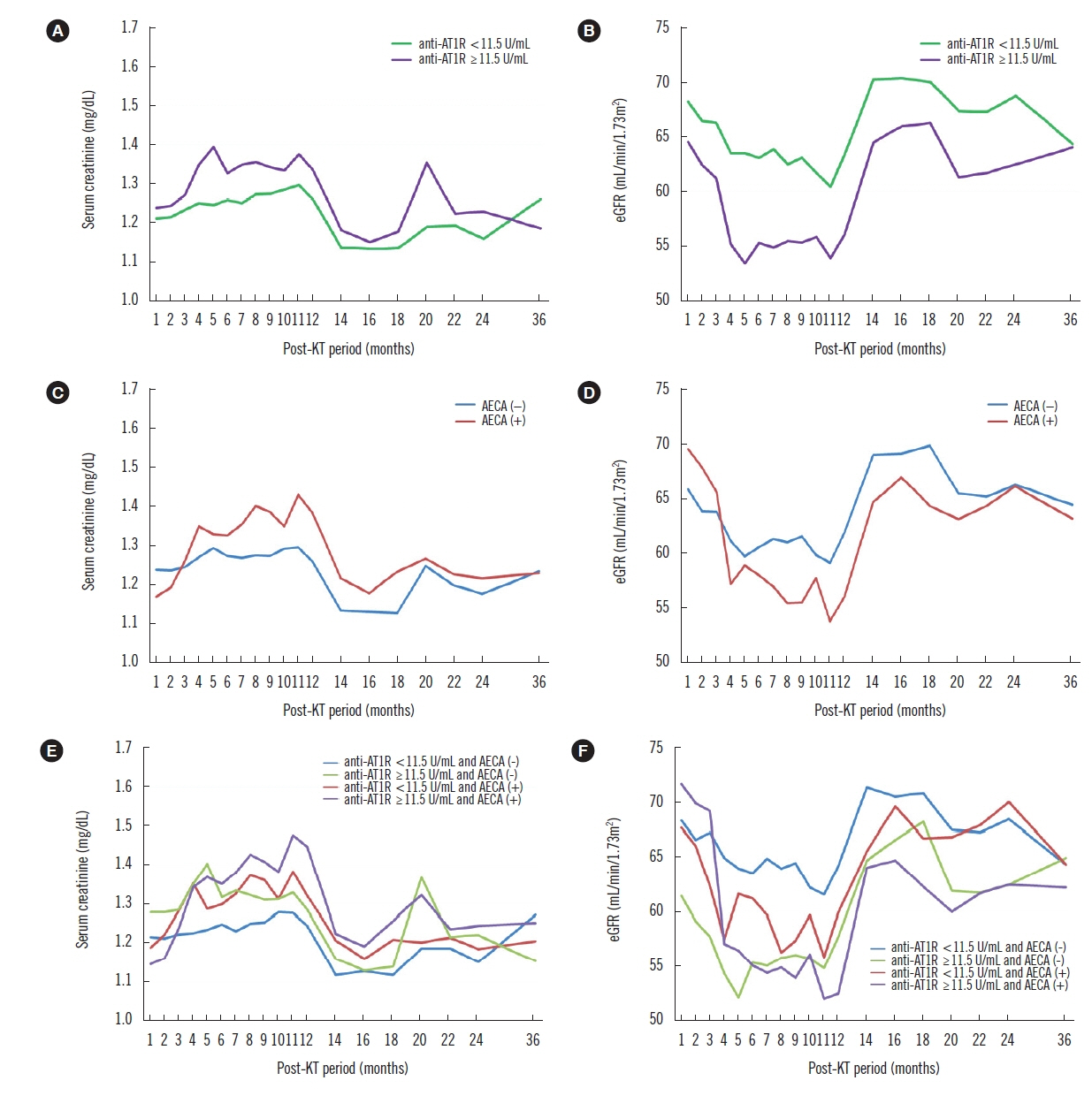
Pre-transplant anti-AT1Rs (≥11.5 U/mL) and AECAs were observed in 36 (38.3%) and 22 recipients (23.4%), respectively; 11 recipients had both. Pre-transplant anti-AT1Rs were a significant risk factor for the development of acute rejection (AR) (HR 2.09; P=0.018), while a positive AECA status was associated with AR or microvascular inflammation only (HR 2.47; P=0.004) throughout the follow-up period. In particular, AECA (+) recipients with ≥11.5 U/mL anti-AT1Rs exhibited a significant effect on creatinine and estimated glomerular filtration rate (P<0.001; P=0.028), although the risk of AR was not significant.
Conclusions
Pre-transplant anti-AT1Rs and AECAs have independent negative effects on post-transplant outcomes in low-risk LDKT recipients. Assessment of both antibodies would be helpful in stratifying the pre-transplant immunological risk, even in low-risk LDKT recipients.
Keyword
Figure
Cited by 2 articles
-
Prediction of HLA-DQ in Deceased Donors and its Clinical Significance in Kidney Transplantation
Soo-Kyung Kim, John Jeongseok Yang, Sang-Hyun Hwang, Heungsup Sung, Sung Shin, Sun-Young Ko, Heung-Bum Oh
Ann Lab Med. 2021;41(2):190-197. doi: 10.3343/alm.2021.41.2.190.Causes of Positive Pretransplant Crossmatches in the Absence of Donor-Specific Anti-Human Leukocyte Antigen Antibodies: A Single-Center Experience
Hyunhye Kang, Jaeeun Yoo, Sang-Yoon Lee, Eun-Jee Oh
Ann Lab Med. 2021;41(4):429-435. doi: 10.3343/alm.2021.41.4.429.
Reference
-
1. Terasaki PI. Humoral theory of transplantation. Am J Transplant. 2003; 3:665–73.
Article2. Opelz G and Collaborative Transplant Study. Non-HLA transplantation immunity revealed by lymphocytotoxic antibodies. Lancet. 2005; 365:1570–6.3. Han F, Lv R, Jin J, Wu J, Chen Y, Wang H, et al. Pre-transplant serum concentrations of anti-endothelial cell antibody in panel reactive antibody negative renal recipients and its impact on acute rejection. Clin Chem Lab Med. 2009; 47:1265–9.
Article4. Breimer ME, Rydberg L, Jackson AM, Lucas DP, Zachary AA, Melancon JK, et al. Multicenter evaluation of a novel endothelial cell crossmatch test in kidney transplantation. Transplantation. 2009; 87:549–56.
Article5. Xavier P, Aires P, Sampaio S, Mendes C, Monteiro M, Alves H, et al. XM-ONE detection of endothelium cell antibodies identifies a subgroup of HLA-antibody negative patients undergoing acute rejection. Transplant Proc. 2011; 43:91–4.
Article6. Ronda C, Borba SC, Ferreira SC, Glotz D, Ianhez LE, Rodrigues H, et al. Non-human leukocyte antigen antibodies reactive with endothelial cells could be involved in early loss of renal allografts. Transplant Proc. 2011; 43:1345–8.
Article7. Taniguchi M, Rebellato LM, Cai J, Hopfield J, Briley KP, Haisch CE, et al. Higher risk of kidney graft failure in the presence of anti-angiotensin II type-1 receptor antibodies. Am J Transplant. 2013; 13:2577–89.
Article8. Giral M, Foucher Y, Dufay A, Van Huyen JP, Renaudin K, Moreau A, et al. Pretransplant sensitization against angiotensin II type 1 receptor is a risk factor for acute rejection and graft loss. Am J Transplant. 2013; 13:2567–76.
Article9. Banasik M, Boratyńska M, Kościelska-Kasprzak K, Kamińska D, Bartoszek D, Zabińska M, et al. The influence of non-HLA antibodies directed against angiotensin II type 1 receptor (AT1R) on early renal transplant outcomes. Transpl Int. 2014; 27:1029–38.
Article10. Banasik M, Boratyńska M, Kościelska-Kasprzak K, Kamińska D, Zmonarski S, Mazanowska O, et al. Non-HLA antibodies: angiotensin II type 1 receptor (anti-AT1R) and endothelin-1 type A receptor (anti-ETAR) are associated with renal allograft injury and graft loss. Transplant Proc. 2014; 46:2618–21.
Article11. Jackson AM, Sigdel TK, Delville M, Hsieh SC, Dai H, Bagnasco S, et al. Endothelial cell antibodies associated with novel targets and increased rejection. J Am Soc Nephrol. 2015; 26:1161–71.
Article12. Philogene MC, Bagnasco S, Kraus ES, Montgomery RA, Dragun D, Leffell MS, et al. Anti-angiotensin II Type 1 receptor and anti-endothelial cell antibodies: A cross-sectional analysis of pathological findings in allograft biopsies. Transplantation. 2017; 101:608–15.13. Philogene MC, Zhou S, Lonze BE, Bagnasco S, Alasfar S, Montgomery RA, et al. Pre-transplant screening for non-HLA antibodies: who should be tested? Hum Immunol. 2018; 79:195–202.
Article14. Piotti G, Palmisano A, Maggiore U, Buzio C. Vascular endothelium as a target of immune response in renal transplant rejection. Front Immunol. 2014; 5:505.
Article15. Fuss A, Hope CM, Deayton S, Bennett GD, Holdsworth R, Carroll RP, et al. C4d-negative antibody-mediated rejection with high anti-angiotensin II type I receptor antibodies in absence of donor-specific antibodies. Nephrology (Carlton). 2015; 20:467–73.
Article16. Min JW, Lee H, Choi BS, Park CW, Yang CW, Kim YS, et al. Clinical impact of pre-transplant antibodies against angiotensin II Type I receptor and major histocompatibility complex class I-related chain A in kidney transplant patients. Ann Lab Med. 2018; 38:450–7.
Article17. Reinsmoen NL, Lai CH, Mirocha J, Cao K, Ong G, Naim M, et al. Increased negative impact of donor HLA-specific together with non-HLA-specific antibodies on graft outcome. Transplantation. 2014; 97:595–601.
Article18. Haas M, Loupy A, Lefaucheur C, Roufosse C, Glotz D, Seron D, et al. The Banff 2017 Kidney Meeting Report: revised diagnostic criteria for chronic active T cell-mediated rejection, antibody-mediated rejection, and prospects for integrative endpoints for next-generation clinical trials. Am J Transplant. 2018; 18:293–307.
Article19. Batal I, Girnita A, Zeevi A, Saab BA, Stockhausen S, Shapiro R, et al. Clinical significance of the distribution of C4d deposits in different anatomic compartments of the allograft kidney. Mod Pathol. 2008; 21:1490–8.
Article20. Gibson IW, Gwinner W, Bröcker V, Sis B, Riopel J, Roberts IS, et al. Peritubular capillaritis in renal allografts: prevalence, scoring system, reproducibility and clinicopathological correlates. Am J Transplant. 2008; 8:819–25.
Article21. Gupta A, Broin PÓ, Bao Y, Pullman J, Kamal L, Ajaimy M, et al. Clinical and molecular significance of microvascular inflammation in transplant kidney biopsies. Kidney Int. 2016; 89:217–25.
Article22. Daniel V, Sadeghi M, Suesal C, Scherer S, Tran H, Gombos P, et al. Clinical relevance of preformed IgG and IgM antibodies against donor endothelial progenitor cells in recipients of living donor kidney grafts. Clin Transplant. 2016; 30:124–30.
Article23. Zitzner JR, Shah S, Jie C, Wegner W, Tambur AR, Friedewald JJ. A prospective study evaluating the role of donor-specific anti-endothelial crossmatch (XM-ONE assay) in predicting living donor kidney transplant outcome. Hum Immunol. 2013; 74:1431–6.
Article24. Gareau AJ, Wiebe C, Pochinco D, Gibson IW, Ho J, Rush DN, et al. Pre-transplant AT1R antibodies correlate with early allograft rejection. Transpl Immunol. 2018; 46:29–35.25. In JW, Park H, Rho EY, Shin S, Park KU, Park MH, et al. Anti-angiotensin type 1 receptor antibodies associated with antibody-mediated rejection in patients without preformed HLA-donor-specific antibody. Transplant Proc. 2014; 46:3371–4.
Article26. Banasik M, Boratyńska M, Kościelska-Kasprzak K, Krajewska M, Mazanowska O, Kamińska D, et al. The impact of non-HLA antibodies directed against endothelin-1 type A receptors (ETAR) on early renal transplant outcomes. Transpl Immunol. 2014; 30:24–9.
Article27. Cuevas E, Arreola-Guerra JM, Hernández-Méndez EA, Salcedo I, Castelán N, Uribe-Uribe NO, et al. Pretransplant angiotensin II type 1-receptor antibodies are a risk factor for earlier detection of de novo HLA donor-specific antibodies. Nephrol Dial Transplant. 2016; 31:1738–45.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Angiotensin II type 1 receptor antibodies in kidney transplantation
- Investigation of Serum Angiotensin II Type 1 Receptor Antibodies at the Time of Renal Allograft Rejection
- Clinical Impact of Pre-transplant Antibodies Against Angiotensin II Type I Receptor and Major Histocompatibility Complex Class I-Related Chain A in Kidney Transplant Patients
- Preexisting nonhuman leukocyte antigen antibodies are associated with allograft rejection after thoracic transplantation
- Association of preoperative non-HLA antibodies with kidney allograft rejection