Neonatal Med.
2020 Feb;27(1):31-36. 10.5385/nm.2020.27.1.31.
Central Diabetes Insipidus in an Extremely-Low-Birth-Weight Preterm Infant with Suspected Ectopic Posterior Lobe of the Pituitary Gland
- Affiliations
-
- 1Department of Pediatrics, Hallym University Kangnam Sacred Heart Hospital, Hallym University College of Medicine, Seoul, Korea
- 2Department of Radiology, Hallym University Kangnam Sacred Heart Hospital, Hallym University College of Medicine, Seoul, Korea
- KMID: 2502091
- DOI: http://doi.org/10.5385/nm.2020.27.1.31
Abstract
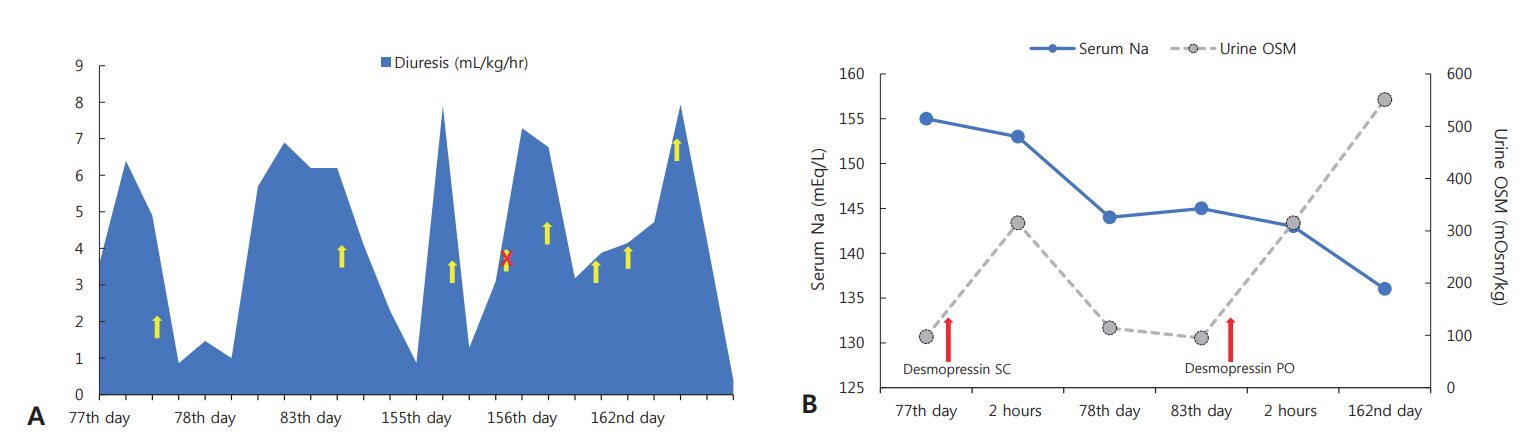
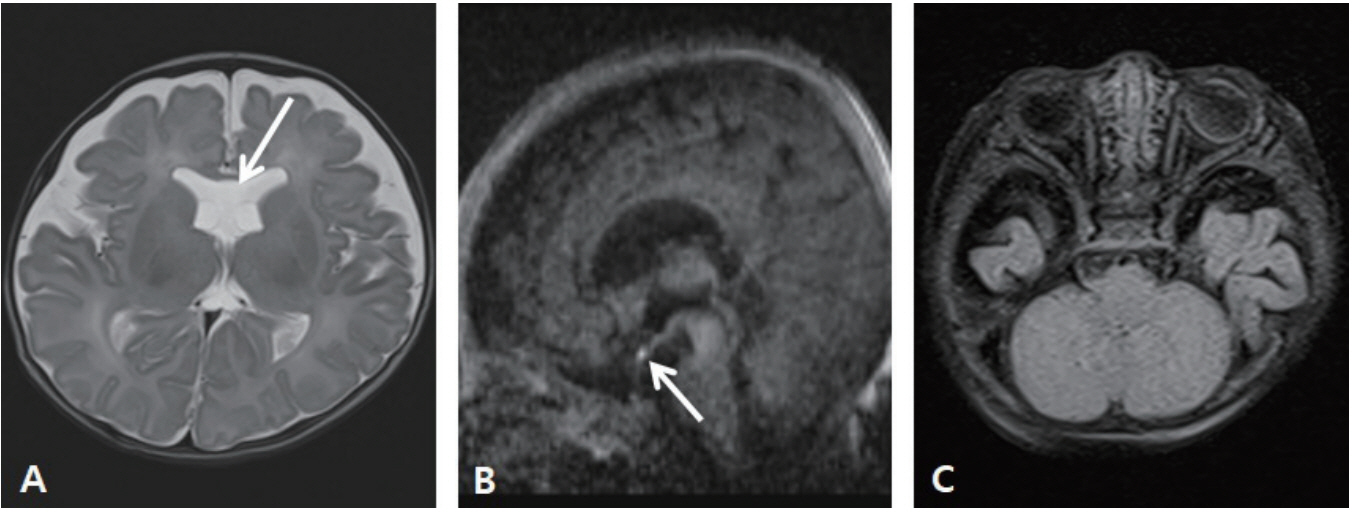
- Central diabetes insipidus (CDI) is extremely rare in neonates, especially in extremely-low-birth-weight infants, and most cases are secondary to conditions, such as ischemic or hemorrhagic brain damage. Here, we report a case of CDI in a 530-g infant born at 23+3 weeks of gestation, with suspected ectopic posterior pituitary gland. Hypernatremia was noticed at 33+6 weeks of postmenstrual age, and it persisted with in creased volumes of diluted urine, despite adequate sodium intake. Serum and urine osmolality returned to the normal range after administration of a desmopressin injection. The bright spot of the posterior pituitary was absent, and brain magnetic resonance imaging suggested an ectopic posterior pituitary gland. At the time of writing this manuscript, the patient was on oral desmopressin medication without complications at the corrected age of 8 months. Through this report, we emphasize that although CDI is extremely rare in premature infants, it should be suspected when hypernatremia and polyuria that are unexplained by other causes are noted.
Keyword
Figure
Reference
-
1. Di Iorgi N, Napoli F, Allegri AE, Olivieri I, Bertelli E, Gallizia A, et al. Diabetes insipidus: diagnosis and management. Horm Res Paediatr. 2012; 77:69–84.2. Biset A, Claris O. Idiopathic central diabetes insipidus in an extreme premature infant: a case report. Arch Pediatr. 2018; 25:480–4.3. Ferlin ML, Sales DS, Celini FP, Martinelli Junior CE. Central diabetes insipidus: alert for dehydration in very low birth weight infants during the neonatal period. A case report. Sao Paulo Med J. 2015; 133:60–3.4. Hanta D, Torer B, Temiz F, Kilicdag H, Gokce M, Erdogan O. Idiopathic central diabetes insipidus presenting in a very low birth weight infant successfully managed with lyophilized sublingual desmopressin. Turk J Pediatr. 2015; 57:90–3.5. Quetin F, Garnier H, Brauner R, Vodovar M, Magny JF. Persistent central diabetes insipidus in a very low birth weight infant. Arch Pediatr. 2007; 14:1321–3.6. Stapleton G, DiGeronimo RJ. Persistent central diabetes insipidus presenting in a very low birth weight infant successfully managed with intranasal dDAVP. J Perinatol. 2000; 20:132–4.7. Sng A, Loke KY, Lim Y. A case of neonatal central diabetes insipidus in a premature infant: challenges in diagnosis and management. Case Rep J. 2018; 2:010.8. Djermane A, Elmaleh M, Simon D, Poidvin A, Carel JC, Leger J. Central diabetes insipidus in infancy with or without hypothalamic adipsic hypernatremia syndrome: early identi fication and outcome. J Clin Endocrinol Metab. 2016; 101:635–43.9. Hong YR. Central diabetes insipidus associated with symptomatic cytomegalovirus infection in an extremely low birth weight infant. J Korean Soc Neonatol. 2012; 19:158–62.10. Kurtoglu S, Ozdemir A, Hatipoglu N. Neonatal hypopituitarism: approaches to diagnosis and treatment. J Clin Res Pediatr Endocrinol. 2019; 11:4–12.11. Maghnie M, Villa A, Arico M, Larizza D, Pezzotta S, Beluffi G, et al. Correlation between magnetic resonance imaging of posterior pituitary and neurohypophyseal function in children with diabetes insipidus. J Clin Endocrinol Metab. 1992; 74:795–800.12. Shin JH, Lee HK, Choi CG, Suh DC, Kim CJ, Hong SK, et al. MR imaging of central diabetes insipidus: a pictorial essay. Korean J Radiol. 2001; 2:222–30.13. Ozata M, Tayfun C, Kurtaran K, Yetkin I, Beyhan Z, Corakci A, et al. Magnetic resonance imaging of posterior pituitary for evaluation of the neurohypophyseal function in idiopathic and autosomal dominant neurohypophyseal diabetes insipidus. Eur Radiol. 1997; 7:1098–102.14. Sundarakumar DK, Farley SA, Smith CM, Maravilla KR, Dighe MK, Nixon JN. Absent cavum septum pellucidum: a review with emphasis on associated commissural abnormalities. Pediatr Radiol. 2015; 45:950–64.15. Webb EA, Dattani MT. Septo-optic dysplasia. Eur J Hum Genet. 2010; 18:393–7.16. Rastogi MV, LaFranchi SH. Congenital hypothyroidism. Orphanet J Rare Dis. 2010; 5:17.17. Bahr V, Franzen N, Oelkers W, Pfeiffer AF, Diederich S. Effect of exogenous glucocorticoid on osmotically stimulated antidiuretic hormone secretion and on water reabsorption in man. Eur J Endocrinol. 2006; 155:845–8.18. Ohta M, Kimura T, Ota K, Shoji M, Inoue M, Sato K, et al. Glucocorticoid-induced central diabetes insipidus in a case of malignant lymphoma. Tohoku J Exp Med. 1991; 163:245–54.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Central Diabetes Insipidus Associated with Symptomatic Cytomegalovirus Infection in an Extremely Low Birth Weight Infant
- A Case of Central Diabetes Insipidus with Growth Hormone Deficiency and Loss of Hyperintense Signal in the Posterior Lobe
- Reappearance of the posterior pituitary bright signal in diabetes insipidus: MR follow-up of germinomas after radiotherapy
- A Case of Teratocarcinoma with Central Diabetes Insipidus
- A Case of Central Diabetes Insipidus Developed during Puerperium