Neurointervention.
2020 Mar;15(1):4-17. 10.5469/neuroint.2019.00213.
Sclerotherapy for Venous Malformations of Head and Neck: Systematic Review and Meta-Analysis
- Affiliations
-
- 1Department of Neurosurgery, Mayo Clinic, Rochester, MN, USA
- 2Humanitas University, Milano, IT, USA
- 3Department of Otorhinolaryngology, Mayo Clinic, Rochester, MN, USA
- 4Department of Dermatology and Pediatrics, Mayo Clinic, Rochester, MN, USA
- 5Department of Radiology and Vascular Centers, Mayo Clinic, Rochester, MN, USA
- KMID: 2502077
- DOI: http://doi.org/10.5469/neuroint.2019.00213
Abstract
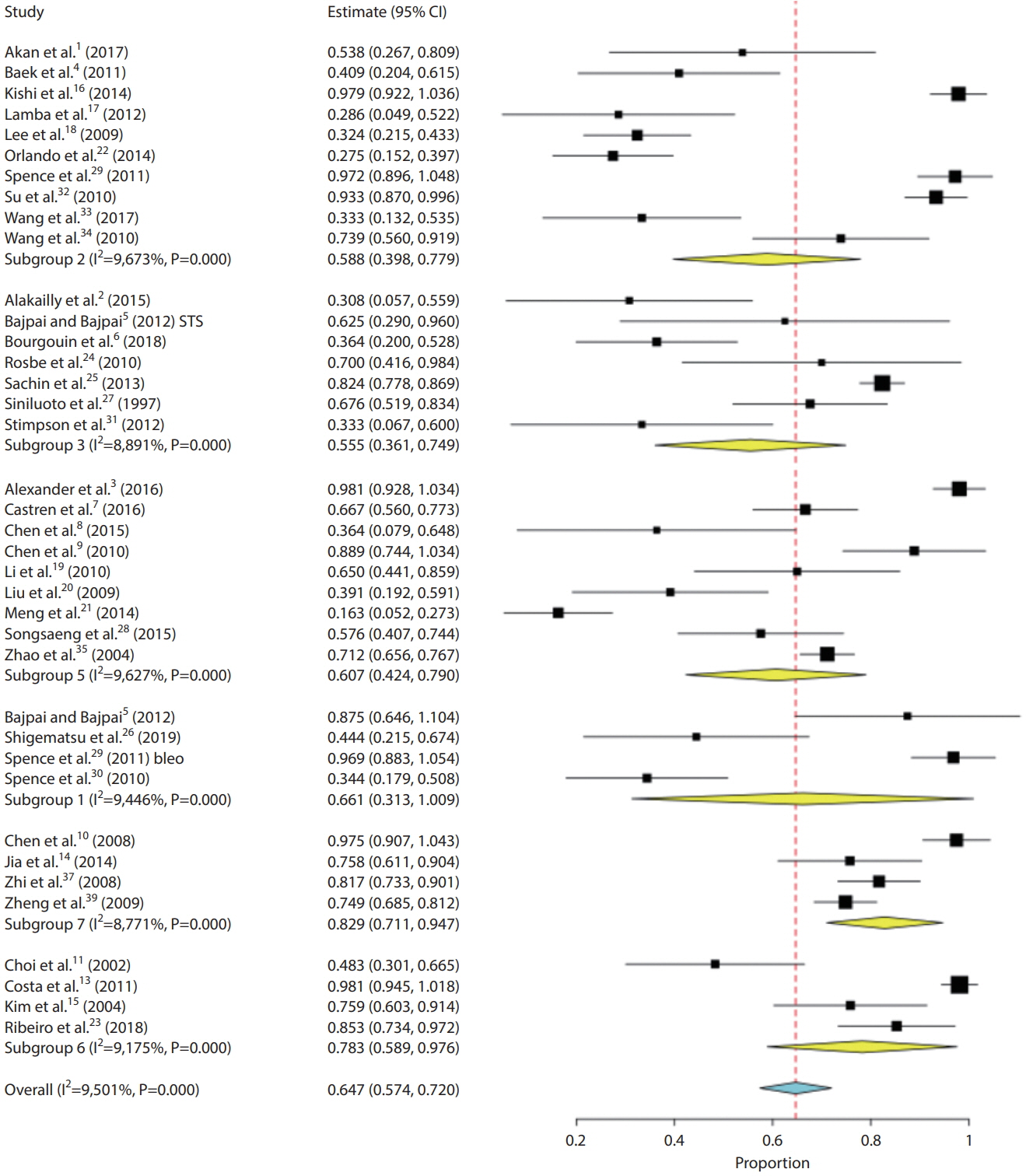
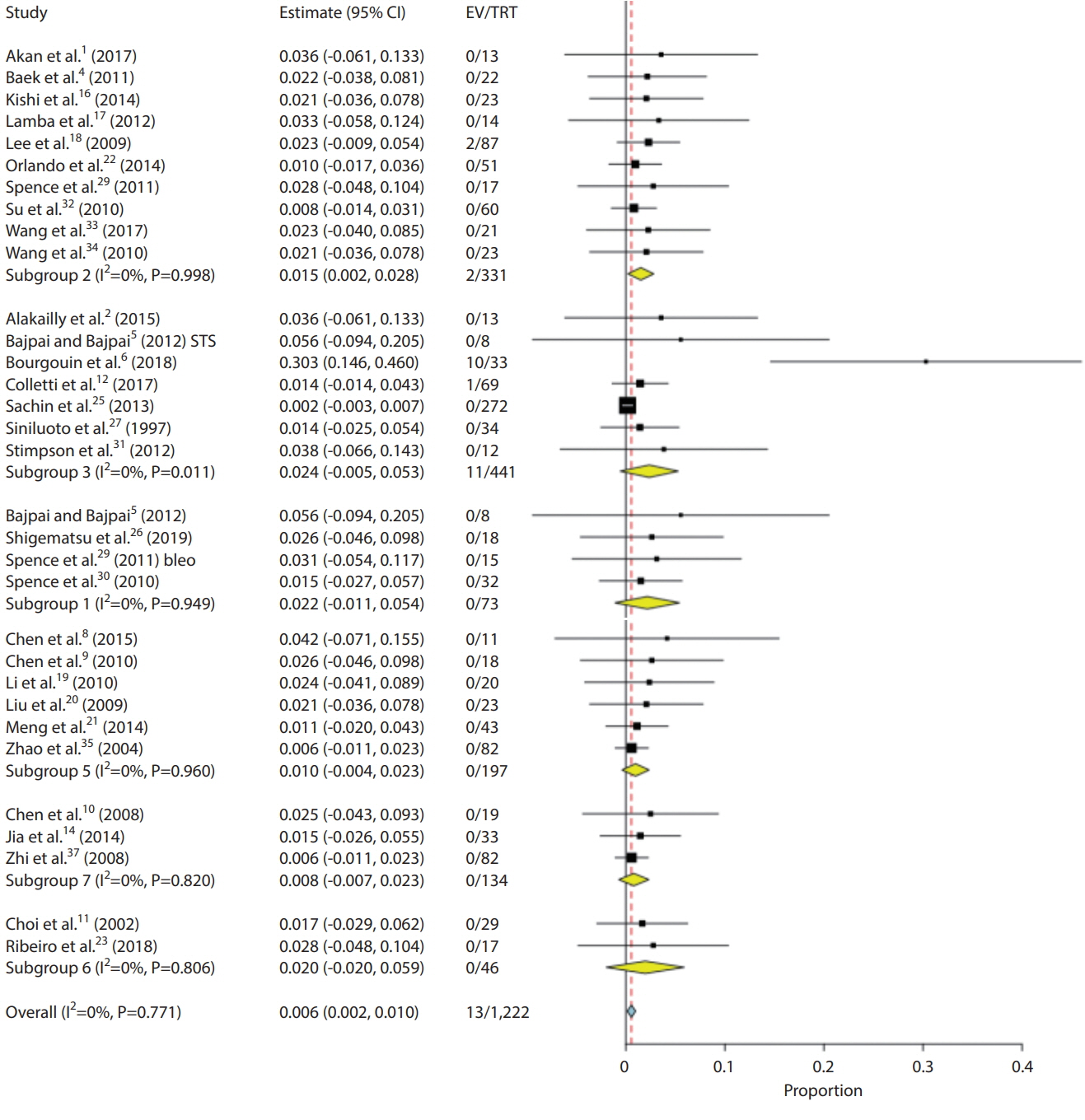
- We performed a systematic review and meta-analysis of studies performing sclerotherapy for treatment of venous malformations (VMs) of the face, head and neck. It is our hope that data from this study could be used to better inform providers and patients regarding the benefits and risks of percutaneous sclerotherapy for treatment of face, head and neck VMs. We searched PubMed, MEDLINE, and EMBASE from 2000–2018 for studies evaluating the safety and efficacy of percutaneous sclerotherapy of neck, face and head VMs. Two independent reviewers selected studies and abstracted data. The primary outcomes were complete and partial resolution of the VM. Data were analyzed using random-effects meta-analysis. Thirty-seven studies reporting on 2,067 patients were included. The overall rate of complete cure following percutaneous sclerotherapy with any agent was 64.7% (95% confidence interval [CI], 57.4–72.0%). Sodium tetradecyl sulfate had the lowest complete cure rate at 55.5% (95% CI, 36.1–74.9%) while pingyangmycin had the highest cure rate at 82.9% (95% CI, 71.1–94.7%). Overall patient satisfaction rates were 91.0% (95% CI, 86.1–95.9%). Overall quality of life improvement was 78.9% (95% CI, 67.0–90.8%). Overall permanent morbidity/mortality was 0.8% (95% CI, 0.3–1.3%) with no cases of mortality. Our systematic review and meta-analysis of 37 studies and over 2,000 patients found that percutaneous sclerotherapy is a very safe and effective treatment modality for treatment of VMs of the head, neck and face.
Keyword
Figure
Reference
-
1. Akan H, Uzunkaya F, Soylu AI. Percutaneous ethanol sclerotherapy of venous malformations of the oral cavity and the oropharynx. Erciyes Med. 2017; 39:183–188.
Article2. Alakailly X, Kummoona R, Quereshy FA, Baur DA, González AE. The use of sodium tetradecyl sulphate for the treatment of venous malformations of the head and neck. J Maxillofac Oral Surg. 2015; 14:332–338.
Article3. Alexander MD, McTaggart RA, Choudhri OA, Pandit RP, Wu A, Ross M, et al. Quantitative volumetric analysis of head and neck venous and lymphatic malformations to assess response to percutaneous sclerotherapy. Acta Radiol. 2016; 57:205–209.
Article4. Baek HJ, Hong JP, Choi JW, Suh DC. Direct percutaneous alcohol sclerotherapy for venous malformations of head and neck region without fluoroscopic guidance: technical consideration and outcome. Neurointervention. 2011; 6:84–88.
Article5. Bajpai H, Bajpai S. Comparative analysis of intralesional sclerotherapy with sodium tetradecyl sulfate versus bleomycin in the management of low flow craniofacial soft tissue vascular lesions. J Maxillofac Oral Surg. 2012; 11:13–20.
Article6. Bourgouin P, Thomas-Chaussé F, Gilbert P, Giroux MF, Périgny S, Guertin L, et al. Effectiveness and safety of sclerotherapy for treatment of low-flow vascular malformations of the oropharyngeal region. J Vasc Interv Radiol. 2018; 29:809–815.
Article7. Castren E, Aronniemi J, Klockars T, Pekkola J, Lappalainen K, Vuola P, et al. Complications of sclerotherapy for 75 head and neck venous malformations. Eur Arch Otorhinolaryngol. 2016; 273:1027–1036.
Article8. Chen AW, Liu YR, Li K, Zhang K, Wang T, Liu SH. Efficacy of sclerotherapy with radio-opaque foam guided by digital subtraction angiography for the treatment of complex venous malformations of the head and neck. Br J Oral Maxillofac Surg. 2015; 53:809–813.
Article9. Chen WL, Huang ZQ, Zhang DM, Chai Q. Percutaneous sclerotherapy of massive venous malformations of the face and neck using fibrin glue combined with ok-432 and pingyangmycin. Head Neck. 2010; 32:467–472.
Article10. Chen Y, Li Y, Zhu Q, Zeng Q, Zhao J, He X, et al. Fluoroscopic intralesional injection with pingyangmycin lipiodol emulsion for the treatment of orbital venous malformations. AJR Am J Roentgenol. 2008; 190:966–971.
Article11. Choi YH, Han MH, O-Ki K, Cha SH, Chang KH. Craniofacial cavernous venous malformations: percutaneous sclerotherapy with use of ethanolamine oleate. J Vasc Interv Radiol. 2002; 13:475–482.12. Colletti G, Deganello A, Bardazzi A, Mattassi R, Dalmonte P, Gazzabin L, et al. Complications after treatment of head and neck venous malformations with sodium tetradecyl sulfate foam. J Craniofac Surg. 2017; 28:e388. –e392.
Article13. Costa JR, Torriani MA, Hosni ES, D’Avila OP, de Figueiredo PJ. Sclerotherapy for vascular malformations in the oral and maxillofacial region: treatment and follow-up of 66 lesions. J Oral Maxillofac Surg. 2011; 69:e88. –e92.14. Jia R, Xu S, Huang X, Song X, Pan H, Zhang L, et al. Pingyangmycin as first-line treatment for low-flow orbital or periorbital venous malformations: evaluation of 33 consecutive patients. JAMA Ophthalmol. 2014; 132:942–948.15. Kim KH, Sung MW, Roh JL, Han MH. Sclerotherapy for congenital lesions in the head and neck. Otolaryngol Head Neck Surg. 2004; 131:307–316.
Article16. Kishi K, Morita N, Terada T, Sato M. Dose-saving isolation procedure in percutaneous ethanol sclerotherapy for venous malformations. Phlebology. 2014; 29:276–286.
Article17. Lamba S, Keshava SKN, Moses V, Surendrababu N, Gupta AK. Ethanol sclerotherapy for treatment of venous malformations of face and neck—a single centre experience. Eur J Plast Surg. 2012; 35:345–350.18. Lee IH, Kim KH, Jeon P, Byun HS, Kim HJ, Kim ST, et al. Ethanol sclerotherapy for the management of craniofacial venous malformations: the interim results. Korean J Radiol. 2009; 10:269–276.
Article19. Li J, Chen J, Zheng G, Liao G, Fu Z, Li J, et al. Digital subtraction angiography-guided percutaneous sclerotherapy of venous malformations with pingyangmycin and/or absolute ethanol in the maxillofacial region. J Oral Maxillofac Surg. 2010; 68:2258–2266.
Article20. Liu Y, Liu D, Wang Y, Zhang W, Zhao F. Clinical study of sclerotherapy of maxillofacial venous malformation using absolute ethanol and pingyangmycin. J Oral Maxillofac Surg. 2009; 67:98–104.
Article21. Meng J, Zhuang QW, Gu QP, Zhang J, Li ZP, Si YM. Digital subtraction angiography (DSA) guided sequential sclerotherapy for maxillofacial vein malformation. Eur Rev Med Pharmacol Sci. 2014; 18:1709–1712.22. Orlando JL, Caldas JG, Campos HG, Nishinari K, Krutman M, Wolosker N. Ethanol sclerotherapy of head and neck venous malformations. Einstein (Sao Paulo). 2014; 12:181–186.
Article23. Ribeiro MC, de Mattos Camargo Grossmann S, do Amaral MBF, de Castro WH, Navarro TP, Procopio RJ, et al. Effectiveness and safety of foam sclerotherapy with 5% ethanolamine oleate in the treatment of low-flow venous malformations in the head and neck region: a case series. Int J Oral Maxillofac Surg. 2018; 47:900–907.
Article24. Rosbe KW, Hess CP, Dowd CF, Frieden IJ. Masseteric venous malformations: diagnosis, treatment, and outcomes. Otolaryngol Head Neck Surg. 2010; 143:779–783.
Article25. Sachin K, Rashmi S, Manish S, Siddhartha W, Uday L. Haemangiomas and venous malformations of the head and neck: a retrospective analysis of endovascular management in 358 patients. Indian J Plast Surg. 2013; 46:109–116.
Article26. Shigematsu T, Sorscher M, Dier EC, Berenstein A. Bleomycin sclerotherapy for eyelid venous malformations as an alternative to surgery or laser therapy. J Neurointerv Surg. 2019; 11:57–61.
Article27. Siniluoto TM, Svendsen PA, Wikholm GM, Fogdestam I, Edström S. Percutaneous sclerotherapy of venous malformations of the head and neck using sodium tetradecyl sulphate (sotradecol). Scand J Plast Reconstr Surg Hand Surg. 1997; 31:145–150.
Article28. Songsaeng D, Churojana A, Khumthong R, Mahiwan L. Comparative outcomes for sclerotherapy of head and neck venous vascular malformation between alcohol and bleomycin. J Med Assoc Thai. 2015; 98:408–413.29. Spence J, Krings T, TerBrugge KG, Agid R. Percutaneous treatment of facial venous malformations: a matched comparison of alcohol and bleomycin sclerotherapy. Head Neck. 2011; 33:125–130.
Article30. Spence J, Krings T, terBrugge KG, da Costa LB, Agid R. Percutaneous sclerotherapy for facial venous malformations: subjective clinical and objective MR imaging follow-up results. AJNR Am J Neuroradiol. 2010; 31:955–960.
Article31. Stimpson P, Hewitt R, Barnacle A, Roebuck DJ, Hartley B. Sodium tetradecyl sulphate sclerotherapy for treating venous malformations of the oral and pharyngeal regions in children. Int J Pediatr Otorhinolaryngol. 2012; 76:569–573.
Article32. Su L, Fan X, Zheng L, Zheng J. Absolute ethanol sclerotherapy for venous malformations in the face and neck. J Oral Maxillofac Surg. 2010; 68:1622–1627.
Article33. Wang D, Su L, Han Y, Wang Z, Zheng L, Li J, et al. Direct intralesional ethanol sclerotherapy of extensive venous malformations with oropharyngeal involvement after a temporary tracheotomy in the head and neck: initial results. Head Neck. 2017; 39:288–296.
Article34. Wang YA, Zheng JW, Zhu HG, Ye WM, He Y, Zhang ZY. Sclerotherapy of voluminous venous malformation in head and neck with absolute ethanol under digital subtraction angiography guidance. Phlebology. 2010; 25:138–144.
Article35. Zhao JH, Zhang WF, Zhao YF. Sclerotherapy of oral and facial venous malformations with use of pingyangmycin and/or sodium morrhuate. Int J Oral Maxillofac Surg. 2004; 33:463–466.
Article36. Zheng JW, Yang XJ, Wang YA, He Y, Ye WM, Zhang ZY. Intralesional injection of pingyangmycin for vascular malformations in oral and maxillofacial regions: an evaluation of 297 consecutive patients. Oral Oncol. 2009; 45:872–876.
Article37. Zhi K, Wen Y, Li L, Ren W. The role of intralesional Pingyangmycin in the treatment of venous malformation of facial and maxillary region. Int J Pediatr Otorhinolaryngol. 2008; 72:593–597.
Article38. Luo QF, Gan YH. Pingyangmycin with triamcinolone acetonide effective for treatment of lymphatic malformations in the oral and maxillofacial region. J Craniomaxillofac Surg. 2013; 41:345–349.
Article39. Shiels WE 2nd, Kang DR, Murakami JW, Hogan MJ, Wiet GJ. Percutaneous treatment of lymphatic malformations. Otolaryngol Head Neck Surg. 2009; 141:219–224.
Article40. Tu JH, Do HM, Patel V, Yeom KW, Teng JMC. Sclerotherapy for lymphatic malformations of the head and neck in the pediatric population. J Neurointerv Surg. 2017; 9:1023–1026.
Article41. Flors L, Leiva-Salinas C, Maged IM, Norton PT, Matsumoto AH, Angle JF, et al. Mr imaging of soft-tissue vascular malformations: diagnosis, classification, and therapy follow-up. Radiographics. 2011; 31:1321–1340. ; discussion 1340-1341.
Article42. Hage AN, Chick JFB, Srinivasa RN, Bundy JJ, Chauhan NR, Acord M, et al. Treatment of venous malformations: the data, where we are, and how it is done. Tech Vasc Interv Radiol. 2018; 21:45–54.
Article43. Albanese G, Kondo KL. Pharmacology of sclerotherapy. Semin Intervent Radiol. 2010; 27:391–399.
Article44. Yesil S, Tanyildiz HG, Bozkurt C, Cakmakci E, Sahin G. Single-center experience with sirolimus therapy for vascular malformations. Pediatr Hematol Oncol. 2016; 33:219–225.
Article45. Klosterman T, O TM. The management of vascular malformations of the airway: natural history, investigations, medical, surgical and radiological management. Otolaryngol Clin North Am. 2018; 51:213–223.46. Sadick M, Wohlgemuth WA, Huelse R, Lange B, Henzler T, Schoenberg SO, et al. Interdisciplinary management of head and neck vascular anomalies: clinical presentation, diagnostic findings and minimalinvasive therapies. Eur J Radiol Open. 2017; 4:63–68.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Venous Malformation of the Pyriform Sinus Treated with Ethanol Sclerotherapy
- Efficacy of Percutaneous Sclerotherapy in Low Flow Venous Malformations - A Single Center Series
- Therapeutic Effect of Sclerotherapy on Venous Malformations
- Surgical approach for venous malformation in the head and neck
- Sclerotherapy of Multiple Intraoral Venous Malformations with Use of Ethanolamine Oleate: A Case Report