J Korean Neurosurg Soc.
2020 Mar;63(2):178-187. 10.3340/jkns.2019.0077.
Vasa Vasorum Densities in Human Carotid Atherosclerosis Is Associated with Plaque Development and Vulnerability
- Affiliations
-
- 1Department of Neurosurgery, Chonnam National University Hospital, Chonnam National University Medical School, Gwangju, Korea
- 2Department of Forensic Medicine, Chonnam National University Hospital, Chonnam National University Medical School, Gwangju, Korea
- KMID: 2501704
- DOI: http://doi.org/10.3340/jkns.2019.0077
Abstract
Objective
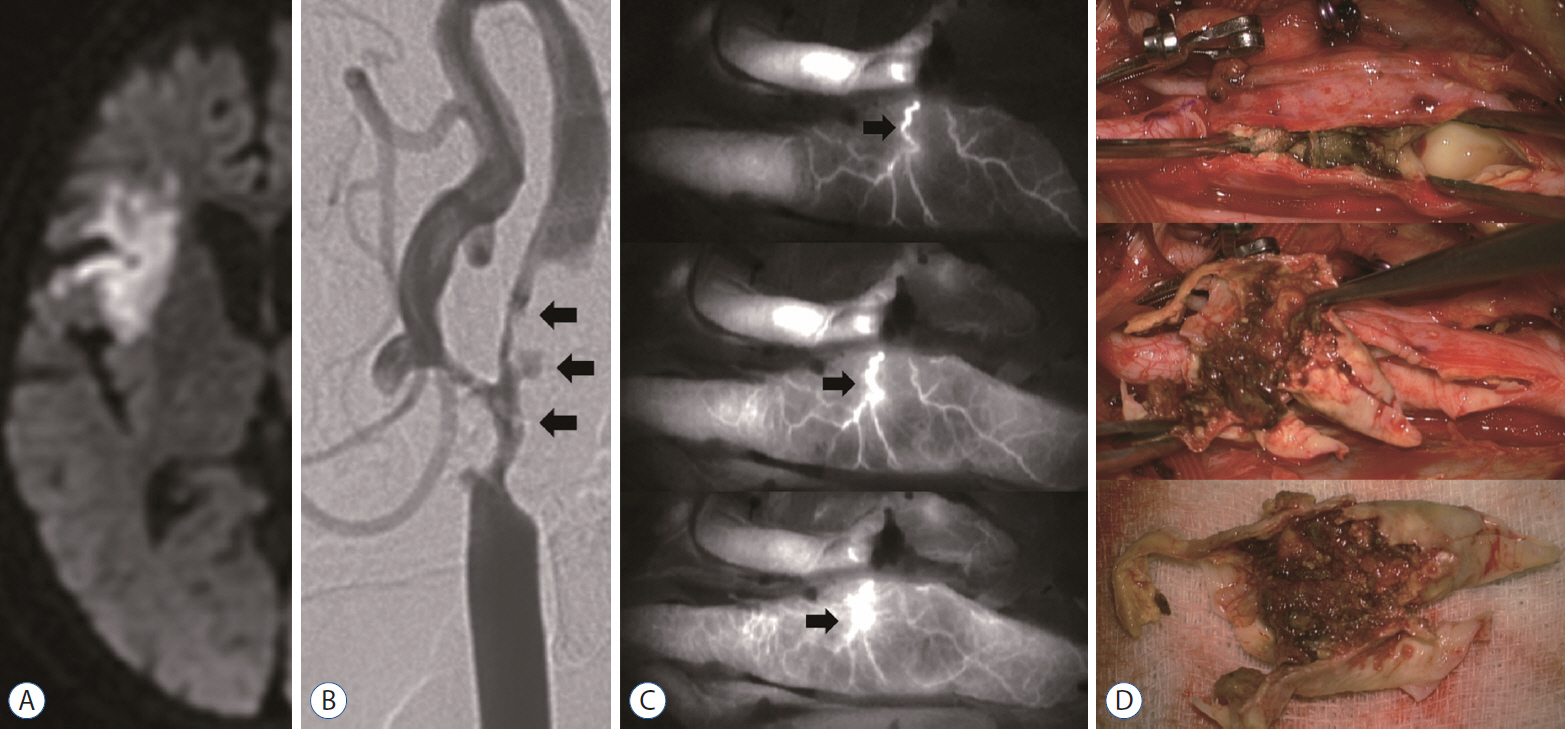
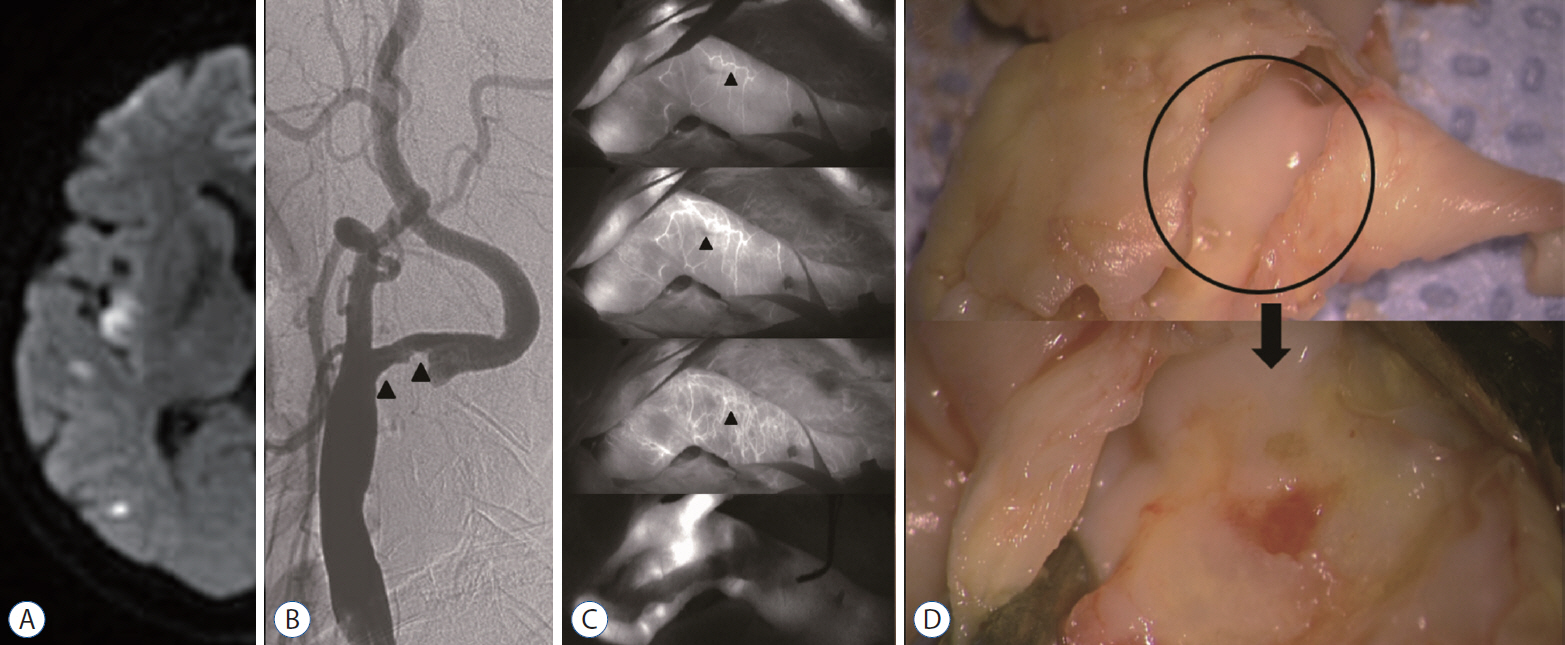
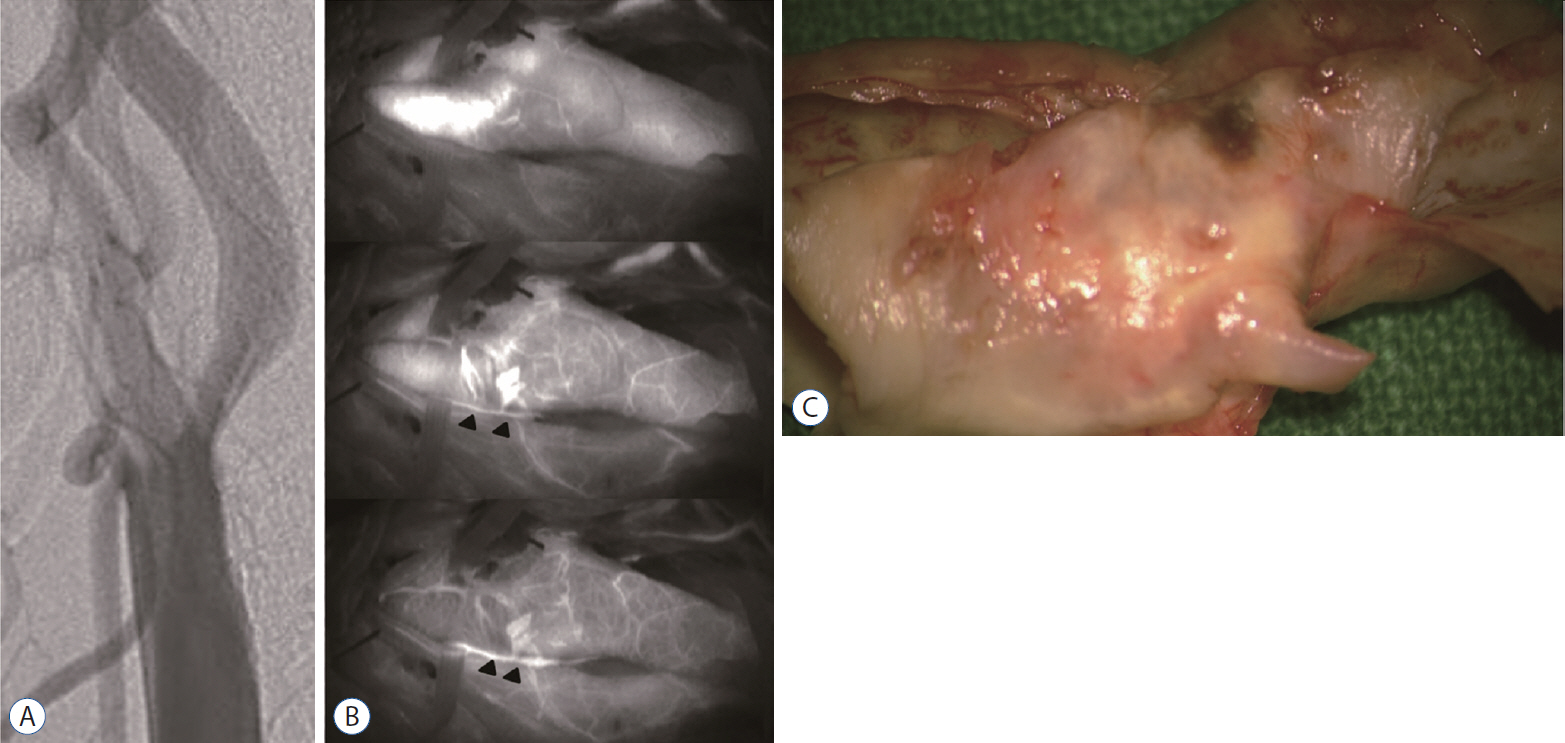
: The extensive vasa vasorum network functions as a conduit for the entry of inflammatory cells or factors that promote the progression of angiogenesis and plaque formation. Therefore, we investigated the correlation between the carotid vasa vasorum activities and carotid plaque vulnerability using indocyanine green video angiography (ICG-VA) during carotid endarterectomy (CEA).
Methods
: Sixty-nine patients who underwent CEA were enrolled prospectively from September 2015 to December 2017. During CEA, a bolus of ICG was injected intravenously before and after resecting the atheroma. Additionally, we performed immunohistochemistry using CD68 (a surface marker of macrophages), CD117 (a surface marker of mast cells), and CD4 and CD8 (surface markers of T-cells) antibodies to analyze the resected plaque specimens.
Results
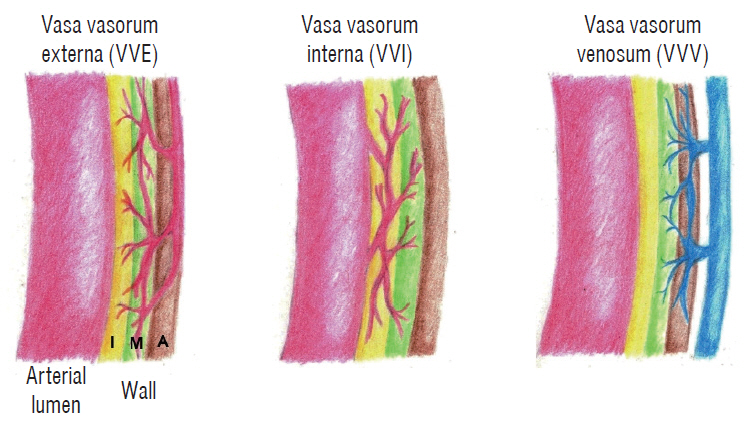
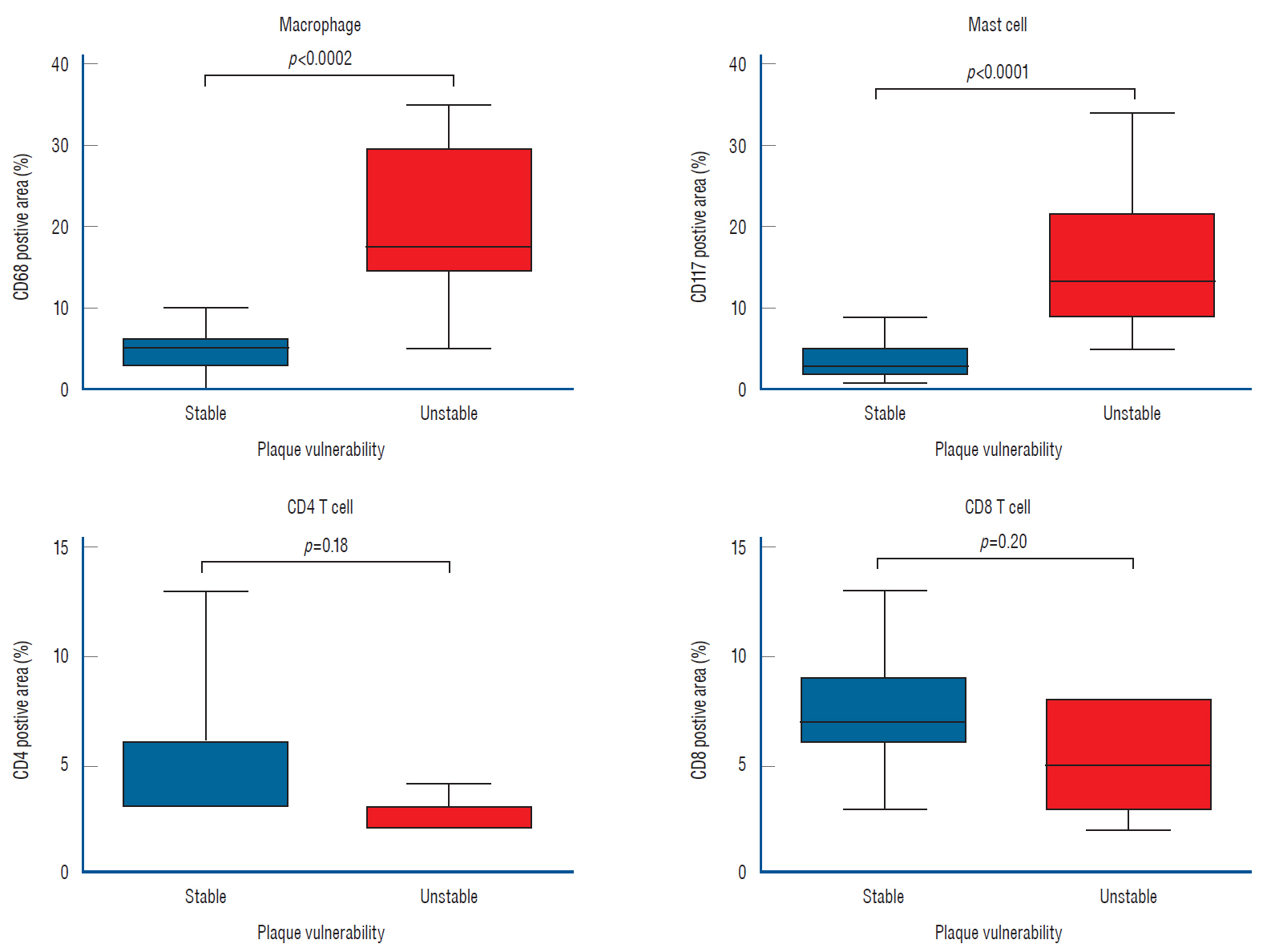
: The density of active vasa vasorum was observed in all patients using ICG-VA. The vasa vasorum externa (VVE) and interna (VVI) were seen in 11 (16%) and 57 patients (82.6%), respectively. Macroscopically, the VVE-type patterns were strongly associated with preoperative angiographic instability (81.8%, p=0.005) and carotid plaque vulnerability (90.9%, p=0.017). In contrast, the VVI-type patterns were weakly associated with angiographic instability (31.6%) and plaque vulnerability (49.1%). CD68-stained macrophages and CD117-stained mast cells were observed more frequently in unstable plaques than in stable plaques (p<0.0001, p=0.002, respectively).
Conclusion
: The early appearance of VVE, along with the presence of many microvessel channels that provided nutrients to the developing and expanding atheroma during ICG-VA, was strongly associated with unstable carotid plaques. The degree of infiltration of macrophages and mast cells is possibly related to the formation of unstable plaques.
Keyword
Figure
Reference
-
References
1. Alonso A, Artemis D, Hennerici MG. Molecular imaging of carotid plaque vulnerability. Cerebrovasc Dis. 39:5–12. 2015.
Article2. Boyle JJ. Association of coronary plaque rupture and atherosclerotic inflammation. J Pathol. 181:93–99. 1997.
Article3. Dashti R, Laakso A, Niemelä M, Porras M, Hernesniemi J. Microscopeintegrated near-infrared indocyanine green videoangiography during surgery of intracranial aneurysms: the Helsinki experience. Surg Neurol. 71:543–550. discussion 550. 2009.
Article4. Doyle B, Caplice N. Plaque neovascularization and antiangiogenic therapy for atherosclerosis. J Am Coll Cardiol. 49:2073–2080. 2007.
Article5. Feinstein SB. Contrast ultrasound imaging of the carotid artery vasa vasorum and atherosclerotic plaque neovascularization. J Am Coll Cardiol. 48:236–243. 2006.
Article6. Fisher M, Paganini-Hill A, Martin A, Cosgrove M, Toole JF, Barnett HJ, et al. Carotid plaque pathology: thrombosis, ulceration, and stroke pathogenesis. Stroke. 36:253–257. 2005.
Article7. Fleiner M, Kummer M, Mirlacher M, Sauter G, Cathomas G, Krapf R, et al. Arterial neovascularization and inflammation in vulnerable patients: early and late signs of symptomatic atherosclerosis. Circulation. 110:2843–2850. 2004.
Article8. Gronholdt ML. Ultrasound and lipoproteins as predictors of lipid-rich, rupture-prone plaques in the carotid artery. Arterioscler Thromb Vasc Biol. 19:2–13. 1999.
Article9. Haga S, Nagata S, Uka A, Akagi Y, Hamada Y, Shono T. Near-infrared indocyanine green videoangiography for assessment of carotid endarterectomy. Acta Neurochir (Wien). 153:1641–1644. discussion 1644. 2011.
Article10. Hansson GK, Libby P, Schönbeck U, Yan ZQ. Innate and adaptive immunity in the pathogenesis of atherosclerosis. Circ Res. 91:281–291. 2002.
Article11. Hiyama T, Tanaka T, Endo S, Komine K, Kudo T, Kobayashi H, et al. Angiogenesis in atherosclerotic plaque obtained from carotid endarterectomy: association between symptomatology and plaque morphology. Neurol Med Chir (Tokyo). 50:1056–1061. 2010.
Article12. Kamiyama K, Nakagawara J, Takada H, Fumoto K, Osato T, Nakamura H. Transformation of the vasa vasorum from normal vessel structures to atheroma nutrient vessels on ICG angiography of cervical carotid artery stenosis. Surg Cereb Stroke (Jpn). 39:413–419. 2011.
Article13. Katano H, Yamada K, Sakurai K, Takahashi S. Depiction of the vasa vasorum during carotid endarterectomy by intraoperative videoangiography. J Stroke Cerebrovasc Dis. 23:2920–2927. 2014.
Article14. Kawahara I, Morikawa M, Honda M, Kitagawa N, Tsutsumi K, Nagata I, et al. High-resolution magnetic resonance imaging using gadolinium-based contrast agent for atherosclerotic carotid plaque. Surg Neurol. 68:60–65. discussion 65-66. 2007.
Article15. Kumamoto M, Nakashima Y, Sueishi K. Intimal neovascularization in human coronary atherosclerosis: its origin and pathophysiological significance. Hum Pathol. 26:450–456. 1995.
Article16. Lane B, Bohnstedt BN, Cohen-Gadol AA. A prospective comparative study of microscope-integrated intraoperative fluorescein and indocyanine videoangiography for clip ligation of complex cerebral aneurysms. J Neurosurg. 122:618–626. 2015.
Article17. Lee CH, Jung YS, Yang HJ, Son YJ, Lee SH. An innovative method for detecting surgical errors using indocyanine green angiography during carotid endarterectomy: a preliminary investigation. Acta Neurochir (Wien). 154:67–73. discussion 73. 2012.
Article18. Lee RT, Libby P. The unstable atheroma. Arterioscler Thromb Vasc Biol. 17:1859–1867. 1997.
Article19. Legein B, Temmerman L, Biessen EA, Lutgens E. Inflammation and immune system interactions in atherosclerosis. Cell Mol Life Sci. 70:3847–3869. 2013.
Article20. Li ZY, Howarth SP, Tang T, Gillard JH. How critical is fibrous cap thickness to carotid plaque stability? A flow-plaque interaction model. Stroke. 37:1195–1199. 2006.
Article21. Libby P. Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol. 32:2045–2051. 2012.
Article22. Libby P. Inflammation in atherosclerosis. Nature. 420:868–874. 2002.
Article23. Libby P, Lichtman AH, Hansson GK. Immune effector mechanisms implicated in atherosclerosis: from mice to humans. Immunity. 38:1092–1104. 2013.
Article24. Mofidi R, Crotty TB, McCarthy P, Sheehan SJ, Mehigan D, Keaveny TV. Association between plaque instability, angiogenesis and symptomatic carotid occlusive disease. Br J Surg. 88:945–950. 2001.
Article25. Mono ML, Karameshev A, Slotboom J, Remonda L, Galimanis A, Jung S, et al. Plaque characteristics of asymptomatic carotid stenosis and risk of stroke. Cerebrovasc Dis. 34:343–350. 2012.
Article26. Moon HS, Joo SP, Seo BR, Jang JW, Kim JH, Kim TS. Value of indocyanine green videoangiography in deciding the completeness of cerebrovascular surgery. J Korean Neurosurg Soc. 53:349–355. 2013.
Article27. Moreno PR, Purushothaman KR, Fuster V, Echeverri D, Truszczynska H, Sharma SK, et al. Plaque neovascularization is increased in ruptured atherosclerotic lesions of human aorta: implications for plaque vulnerability. Circulation. 110:2032–2038. 2004.
Article28. Moulton KS, Vakili K, Zurakowski D, Soliman M, Butterfield C, Sylvin E, et al. Inhibition of plaque neovascularization reduces macrophage accumulation and progression of advanced atherosclerosis. Proc Natl Acad Sci U S A. 100:4736–4741. 2003.
Article29. North American Symptomatic Carotid Endarterectomy Trial Collaborators, Barnett HJM, Taylor DW, Haynes RB, Sackett DL, Peerless SJ, et al. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med. 325:445–453. 1991.
Article30. Ritman EL, Lerman A. The dynamic vasa vasorum. Cardiovasc Res. 75:649–658. 2007.
Article31. Roessler K, Krawagna M, Dörfler A, Buchfelder M, Ganslandt O. Essentials in intraoperative indocyanine green videoangiography assessment for intracranial aneurysm surgery: conclusions from 295 consecutively clipped aneurysms and review of the literature. Neurosurg Focus. 36:E7. 2014.
Article32. Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 340:115–126. 1999.33. Schoenenberger F, Mueller A. On the vascularization of the bovine aortic wall. Helv Physiol Pharmacol Acta. 18:136–150. 1960.34. Shah F, Balan P, Weinberg M, Reddy V, Neems R, Feinstein M, et al. Contrast-enhanced ultrasound imaging of atherosclerotic carotid plaque neovascularization: a new surrogate marker of atherosclerosis? Vasc Med. 12:291–297. 2007.
Article35. Staub D, Schinkel AF, Coll B, Coli S, van der Steen AF, Reed JD, et al. Contrast-enhanced ultrasound imaging of the vasa vasorum: from early atherosclerosis to the identification of unstable plaques. JACC Cardiovasc Imaging. 3:761–771. 2010.36. Weber C, Noels H. Atherosclerosis: current pathogenesis and therapeutic options. Nat Med. 17:1410–1422. 2011.
Article37. Willems S, Vink A, Bot I, Quax PH, de Borst GJ, de Vries JP, et al. Mast cells in human carotid atherosclerotic plaques are associated with intraplaque microvessel density and the occurrence of future cardiovascular events. Eur Heart J. 34:3699–3706. 2013.
Article38. Yamada K, Yoshimura S, Kawasaki M, Enomoto Y, Asano T, Hara A, et al. Embolic complications after carotid artery stenting or carotid endarterectomy are associated with tissue characteristics of carotid plaques evaluated by magnetic resonance imaging. Atherosclerosis. 215:399–404. 2011.
Article39. Yoshida K, Narumi O, Chin M, Inoue K, Tabuchi T, Oda K, et al. Characterization of carotid atherosclerosis and detection of soft plaque with use of black-blood MR imaging. AJNR Am J Neuroradiol. 29:868–874. 2008.
Article40. Zhang Y, Cliff WJ, Schoefl GI, Higgins G. Immunohistochemical study of intimal microvessels in coronary atherosclerosis. Am J Pathol. 143:164–172. 1993.41. Endarterectomy for asymptomatic carotid artery stenosis. Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. JAMA. 273:1421–1428. 1995.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Adventitial Vasa Vasorum in Coronary Artery Diseases
- Capillary Plexus and Vasa of the Rat Lung as Revealed by Scanning Electron Microscope of Corrosion Casts
- General principles of carotid Doppler ultrasonography
- Diagnostic and Therapeutic Approach of Carotid and Cerebrovascular Plaque on the Basis of Vessel Imaging
- Expression of leptin receptor (Ob-R) in human atherosclerotic lesions: potential role in intimal neovascularization