J Pathol Transl Med.
2020 May;54(3):246-252. 10.4132/jptm.2020.02.22.
Continuous quality improvement program and its results of Korean Society for Cytopathology
- Affiliations
-
- 1The Committee of Quality Improvement of Korean Society for Cytopathology, Seoul, Korea
- 2Department of Pathology, Chonnam National University Medical School, Gwangju, Korea
- 3Department of Pathology, Daegu Catholic University School of Medicine, Daegu, Korea
- 4Department of Pathology, Seegene Medical Foundation, Busan, Korea
- 5Department of Pathology, Chungnam National University School of Medicine, Daejeon, Korea
- 6Department of Pathology and Translational Genomics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 7Department of Pathology, National Medical Center, Seoul, Korea
- 8Department of Pathology, Fatima Hospital, Daegu, Korea
- 9Department of Pathology, National Health Insurance Service, Ilsan Hospital, Goyang, Korea
- 10Department of Pathology, Seoul Clinical Laboratories, Seoul, Korea
- 11Department of Pathology, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea
- 12Department of Pathology, Hallym University Dongtan Sacred Heart Hospital, Hwaseong, Korea
- 13Department of Hospital Pathology, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 14Department of Pathology, Sure Quest Lab, Yongin, Korea
- 15Department of Pathology, Hanyang University Guri Hospital, Hanyang University College of Medicine, Guri, Korea
- 16Department of pathology, Dankook University Hospital, Cheonan, Korea
- 17Department of Pathology, Ewha Laboratory of Medicine, Seoul, Korea
- 18Department of Pathology, Pusan National University Hospital, Busan, Korea
- 19Department of Pathology, Busan Paik Hospital, Inje University, Busan, Korea
- KMID: 2501660
- DOI: http://doi.org/10.4132/jptm.2020.02.22
Abstract
- Background
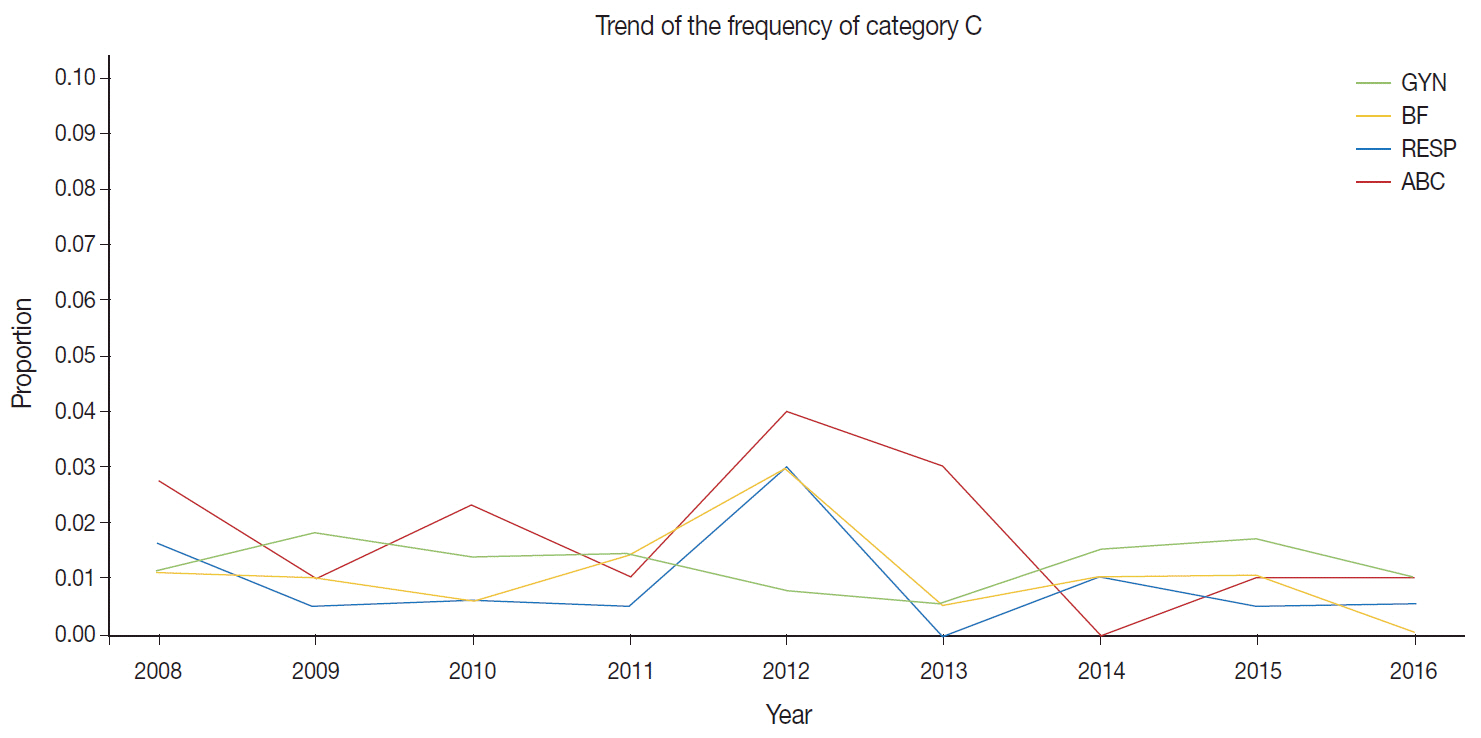
Since 1995, the Korean Society for Cytopathology has overseen the Continuous Quality Improvement program for cytopathology laboratories. The Committee of Quality Improvement has carried out an annual survey of cytology data for each laboratory and set standards for proficiency tests. Methods: Evaluations were conducted four times per year from 2008 to 2018 and comprised statistics regarding cytology diagnoses of previous years, proficiency tests using cytology slides provided by the committee, assessment of adequacy of gynecology (GYN) cytology slides, and submission of cytology slides for proficiency tests. Results: A total of 206 institutes participated in 2017, and the results were as follows. The number of cytology tests increased from year to year. The ratio of liquid-based cytology in GYN gradually decreased, as most of the GYN cytology had been performed at commercial laboratories. The distribution of GYN diagnoses demonstrated nearly 3.0% as atypical squamous cells. The rate for squamous cell carcinoma was less than 0.02%. The atypical squamous cell/squamous intraepithelial lesion ratio was about 3:1 and showed an upward trend. The major discordant rate of cytology-histology in GYN cytology was less than 1%. The proficiency test maintained a major discordant rate less than 2%. The rate of inappropriate specimens for GYN cytology slides gradually decreased. Conclusions: The Continuous Quality Improvement program should be included in quality assurance programs. Moreover, these data can contribute to development of national cancer examination guidelines and facilitate cancer prevention and treatment.
Keyword
Figure
Cited by 2 articles
-
Re-Increasing Trends in Thyroid Cancer Incidence after a Short Period of Decrease in Korea: Reigniting the Debate on Ultrasound Screening
Chan Kwon Jung, Ja Seong Bae, Young Joo Park
Endocrinol Metab. 2022;37(5):816-818. doi: 10.3803/EnM.2022.1586.Current status of cytopathology practice in Korea: impact of the coronavirus pandemic on cytopathology practice
Soon Auck Hong, Haeyoen Jung, Sung Sun Kim, Min-Sun Jin, Jung-Soo Pyo, Ji Yun Jeong, Younghee Choi, Gyungyub Gong, Yosep Chong
J Pathol Transl Med. 2022;56(6):361-369. doi: 10.4132/jptm.2022.09.21.
Reference
-
1. Lee HK, Kim SN, Khang SK, Kang CS, Yoon HK. Quality control program and its results of Korean Society for Cytopathologists. Korean J Cytopathol. 2008; 19:65–71.
Article2. Oh EJ, Jung CK, Kim DH, et al. Current cytology practices in Korea: a nationwide survey by the Korean Society for Cytopathology. J Pathol Transl Med. 2017; 51:579–87.
Article3. Solomon D, Davey D, Kurman R, et al. The 2001 Bethesda System: terminology for reporting results of cervical cytology. JAMA. 2002; 287:2114–9.
Article4. Cibas ES, Ali SZ. The Bethesda System for Reporting Thyroid Cytopathology. Thyroid. 2009; 19:1159–65.
Article5. Fadda G, Rossi ED. Liquid-based cytology in fine-needle aspiration biopsies of the thyroid gland. Acta Cytol. 2011; 55:389–400.
Article6. Chong Y, Ji SJ, Kang CS, Lee EJ. Can liquid-based preparation substitute for conventional smear in thyroid fine-needle aspiration? A systematic review based on meta-analysis. Endocr Connect. 2017; 6:817–29.
Article7. Kim Y, Jun JK, Choi KS, Lee HY, Park EC. Overview of the National Cancer screening programme and the cancer screening status in Korea. Asian Pac J Cancer Prev. 2011; 12:725–30.8. Confortini M, Carozzi F, Dalla Palma P, et al. Interlaboratory reproducibility of atypical squamous cells of undetermined significance report: a national survey. Cytopathology. 2003; 14:263–8.
Article9. Davey DD, Neal MH, Wilbur DC, Colgan TJ, Styer PE, Mody DR. Bethesda 2001 implementation and reporting rates: 2003 practices of participants in the College of American Pathologists Interlaboratory Comparison Program in Cervicovaginal Cytology. Arch Pathol Lab Med. 2004; 128:1224–9.
Article10. Nascimento AF, Cibas ES. The ASC/SIL ratio for cytopathologists as a quality control measure: a follow-up study. Am J Clin Pathol. 2007; 128:653–6.11. Renshaw AA, Deschênes M, Auger M. ASC/SIL ratio for cytotechnologists: a surrogate marker of screening sensitivity. Am J Clin Pathol. 2009; 131:776–81.12. Krieger P, Naryshkin S. Random rescreening of cytologic smears: a practical and effective component of quality assurance programs in both large and small cytology laboratories. Acta Cytol. 1994; 38:291–8.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Current status of cytopathology practice in Korea: impact of the coronavirus pandemic on cytopathology practice
- Current status of cytopathology practices in Korea: annual report on the Continuous Quality Improvement program of the Korean Society for Cytopathology for 2018
- Updates on the Facilities, Procedures, and Performance of the Accredited Endoscopy Unit
- Current state of cytopathology residency training: a Korean national survey of pathologists
- Quality Control Program and Its Results of Korean Society for Cytopathologists