J Pathol Transl Med.
2022 Nov;56(6):361-369. 10.4132/jptm.2022.09.21.
Current status of cytopathology practice in Korea: impact of the coronavirus pandemic on cytopathology practice
- Affiliations
-
- 1Department of Pathology, Chung-Ang University College of Medicine, Seoul, Korea
- 2Department of Pathology, Eone Reference Laboratory, Incheon, Korea
- 3Department of Pathology, Chonnam National University Medical School, Gwangju, Korea
- 4Department of Hospital Pathology, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 5Department of Pathology, Uijeongbu Eulji University Hospital, Eulji University School of Medicine, Uijeongbu, Korea
- 6Department of Pathology, Kyungpook National University Chilgok Hospital, School of Medicine, Kyungpook National University, Daegu, Korea
- 7Department of Pathology, Hallym University Dongtan Sacred Heart Hospital, Hwasung, Korea
- 8Department of Pathology, Asan Medical Center, Ulsan University College of Medicine, Seoul, Korea
- KMID: 2535856
- DOI: http://doi.org/10.4132/jptm.2022.09.21
Abstract
- Background
The Continuous Quality Improvement program for cytopathology in 2020 was completed during the coronavirus pandemic. In this study, we report the result of the quality improvement program.
Methods
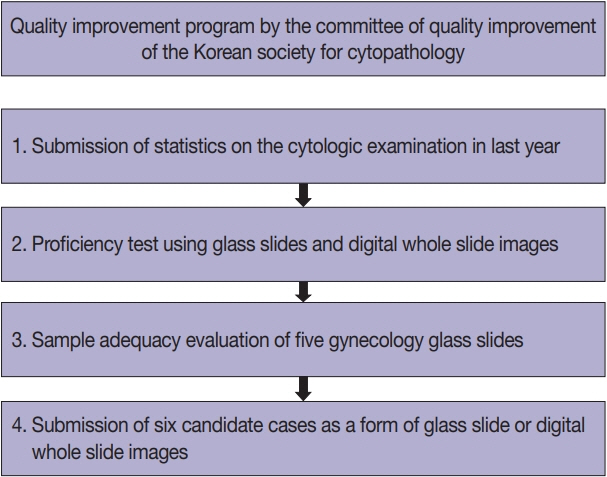
Data related to cytopathology practice from each institute were collected and processed at the web-based portal. The proficiency test was conducted using glass slides and whole-slide images (WSIs). Evaluation of the adequacy of gynecology (GYN) slides from each institution and submission of case glass slides and WSIs for the next quality improvement program were performed.
Results
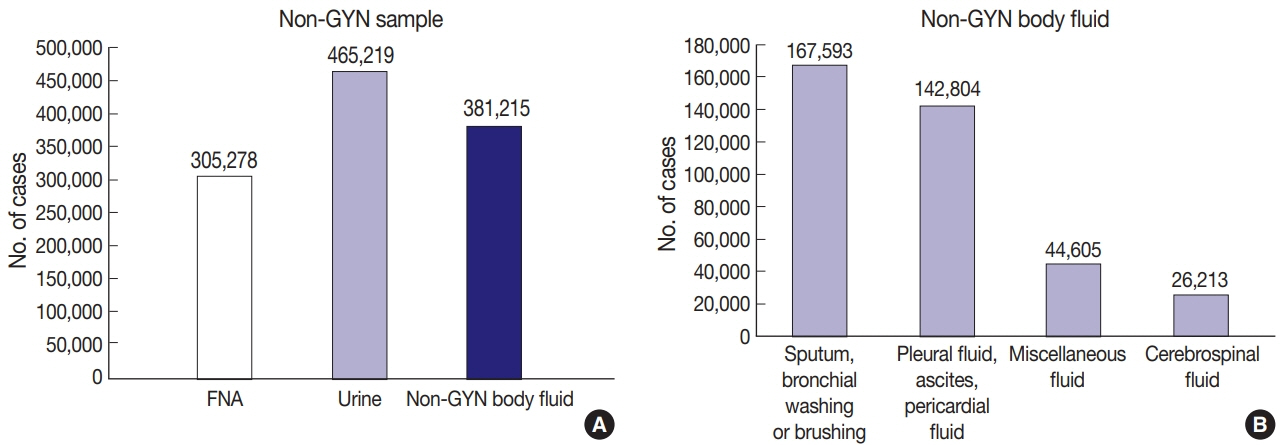
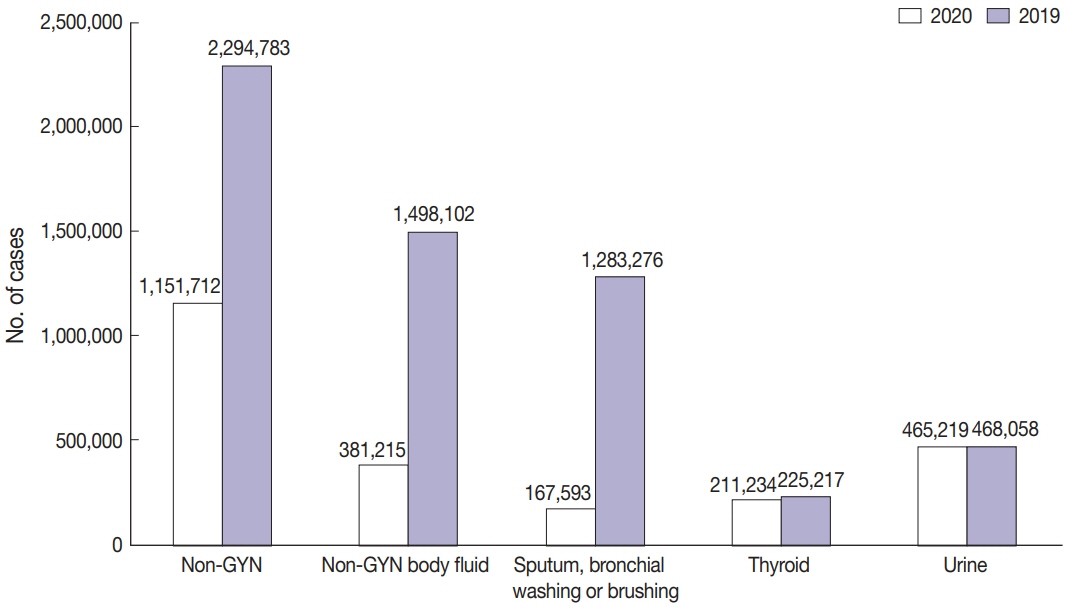
A total of 214 institutions participated in the annual cytopathology survey in 2020. The number of entire cytopathology specimens was 8,220,650, a reduction of 19.0% from the 10,111,755 specimens evaluated in 2019. Notably, the number of respiratory cytopathology specimens, including sputum and bronchial washing/ brushing significantly decreased by 86.9% from 2019, which could be attributed to the global pandemic of coronavirus disease. The ratio of cases with atypical squamous cells to squamous intraepithelial lesions was 4.10. All participating institutions passed the proficiency test and the evaluation of adequacy of GYN slides.
Conclusions
Through the Continuous Quality Improvement program, the effect of coronavirus disease 2019 pandemic, manifesting with a reduction in the number of cytologic examinations, especially in respiratory-related specimen has been identified. The Continuous Quality Improvement Program of the Korean Society for Cytopathology can serve as the gold standard to evaluate the current status of cytopathology practice in Korea.
Keyword
Figure
Reference
-
References
1. Choi YD, Oh HK, Kim SJ, et al. Continuous quality improvement program and its results of Korean Society for Cytopathology. J Pathol Transl Med. 2020; 54:246–52.
Article2. Chong Y, Jung H, Pyo JS, Hong SW, Oh HK. Current status of cytopathology practices in Korea: annual report on the Continuous Quality Improvement program of the Korean Society for Cytopathology for 2018. J Pathol Transl Med. 2020; 54:318–31.
Article3. Lee HK, Kim SN, Khang SK, Kang CS, Yoon HK. Quality control program and its results of Korean Society for Cytopathologists. Korean J Cytopathol. 2008; 19:65–71.
Article4. Oh EJ, Jung CK, Kim DH, et al. Current cytology practices in Korea: a nationwide survey by the Korean Society for Cytopathology. J Pathol Transl Med. 2017; 51:579–87.
Article5. Kim Y, Jun JK, Choi KS, Lee HY, Park EC. Overview of the national cancer screening programme and the cancer screening status in Korea. Asian Pac J Cancer Prev. 2011; 12:725–30.6. Johns Hopkins University of Medicine, Coronavirus Resource Center [Internet]. Baltimore: Johns Hopkins University of Medicine;2022. [cited 2022 Aug 10]. Available from: https://coronavirus.jhu.edu/.7. Moynihan R, Sanders S, Michaleff ZA, et al. Impact of COVID-19 pandemic on utilisation of healthcare services: a systematic review. BMJ Open. 2021; 11:e045343.
Article8. Ministry of Health and Welfare. Yearbook of health and welfare statistics. Sejong: Ministry of Health and Welfare;2020.9. The rate of National Cancer Screening Program in E-national indicator [Internet]. Seoul: Ministry of Health and Welfare;2022. [cited 2022 Aug 10]. Available from: https://www.index.go.kr/potal/main/EachDtlPageDetail.do?idx_cd=1440¶m=002.10. Cox JT. Liquid-based cytology: evaluation of effectiveness, cost-effectiveness, and application to present practice. J Natl Compr Canc Netw. 2004; 2:597–611.
Article11. Dowie R, Stoykova B, Crawford D, et al. Liquid-based cytology can improve efficiency of cervical smear readers: evidence from timing surveys in two NHS cytology laboratories. Cytopathology. 2006; 17:65–72.
Article12. Davey DD, Woodhouse S, Styer P, Stastny J, Mody D. Atypical epithelial cells and specimen adequacy: current laboratory practices of participants in the college of American pathologists interlaboratory comparison program in cervicovaginal cytology. Arch Pathol Lab Med. 2000; 124:203–11.13. Nascimento AF, Cibas ES. The ASC/SIL ratio for cytopathologists as a quality control measure: a follow-up study. Am J Clin Pathol. 2007; 128:653–6.
Article14. Chebib I, Rao RA, Wilbur DC, Tambouret RH. Using the ASC:SIL ratio, human papillomavirus, and interobserver variability to assess and monitor cytopathology fellow training performance. Cancer Cytopathol. 2013; 121:638–43.
Article15. Nayar R, Wilbur DC. The Bethesda system for reporting cervical cytology: definitions, criteria, and explanatory notes. Cham: Springer;2015.16. Pantanowitz L. Digital cytology: look how much has been achieved. Cytopathology. 2020; 31:370–1.
Article17. Eccher A, Girolami I. Current state of whole slide imaging use in cytopathology: pros and pitfalls. Cytopathology. 2020; 31:372–8.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Current state of cytopathology residency training: a Korean national survey of pathologists
- Clinical Practice Experience of Nursing Students During the COVID-19 Pandemic
- Current status of cytopathology practices in Korea: annual report on the Continuous Quality Improvement program of the Korean Society for Cytopathology for 2018
- The Korean Journal of Cytopathology: From Foundation to Unification with the Korean Journal of Pathology
- Impacts of alternative clinical practice on nursing professionalism in nursing students during the COVID-19 pandemic