Standardized Pathology Report for Colorectal Cancer, 2nd Edition
- Affiliations
-
- 1Department of Pathology, Korea University Guro Hospital, Seoul, Korea
- 2Department of Pathology, Inha University School of Medicine, Incheon, Korea
- 3Department of Pathology, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea
- 4Department of Pathology, Research Institute and Hospital, National Cancer Center, Goyang, Korea
- 5Department of Pathology, Eulji University Hospital, Eulji University School of Medicine, Daejeon, Korea
- 6Department of Pathology, School of Medicine, Kyungpook National University, Daegu, Korea
- 7Department of Pathology, Chonbuk National University Medical School, Jeonju, Korea
- 8Department of Pathology, Seoul Paik Hospital, Inje University College of Medicine, Seoul, Korea
- 9Department of Pathology, Chonnam National University Medical School, Gwangju, Korea
- 10Department of Pathology, Yonsei University Wonju College of Medicine, Wonju, Korea
- 11Department of Pathology, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea
- 12Department of Pathology, Seoul National University, Bundang Hospital, Seongnam, Korea
- 13Department of Pathology, Kosin University College of Medicine, Busan, Korea
- 14Department of Pathology, Pusan National University Hospital, Pusan National University School of Medicine, Busan, Korea
- 15Department of Pathology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 16Department of Pathology, Seoul National University Boramae Hospital, Seoul National University College of Medicine, Seoul, Korea
- 17Department of Hospital Pathology, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 18Department of Pathology, Soonchunhyang University Seoul Hospital, Soonchunhyang UniversityCollege of Medicine, Seoul, Korea
- 19Department of Pathology, Konkuk University Medical Center, Konkuk University School of Medicine, Seoul, Korea
- 20Department of Pathology, Kyung Hee University College of Medicine, Seoul, Korea
- KMID: 2501591
- DOI: http://doi.org/10.4132/jptm.2019.09.28
Abstract
- The first edition of the ‘Standardized Pathology Report for Colorectal Cancer,’ which was developed by the Gastrointestinal Pathology Study Group (GIP) of the Korean Society of Pathologists, was published 13 years ago. Meanwhile, there have been many changes in the pathologic diagnosis of colorectal cancer (CRC), pathologic findings included in the pathology report, and immunohistochemical and molecular pathology required for the diagnosis and treatment of colorectal cancer. In order to reflect these changes, we (GIP) decided to make the second edition of the report. The purpose of this standardized pathology report is to provide a practical protocol for Korean pathologists, which could help diagnose and treat CRC patients. This report consists of “standard data elements” and “conditional data elements.” Basic pathologic findings and parts necessary for prognostication of CRC patients are classified as “standard data elements,” while other prognostic factors and factors related to adjuvant therapy are classified as “conditional data elements” so that each institution could select the contents according to the characteristics of the institution. The Korean version is also provided separately so that Korean pathologists can easily understand and use this report. We hope that this report will be helpful in the daily practice of CRC diagnosis.
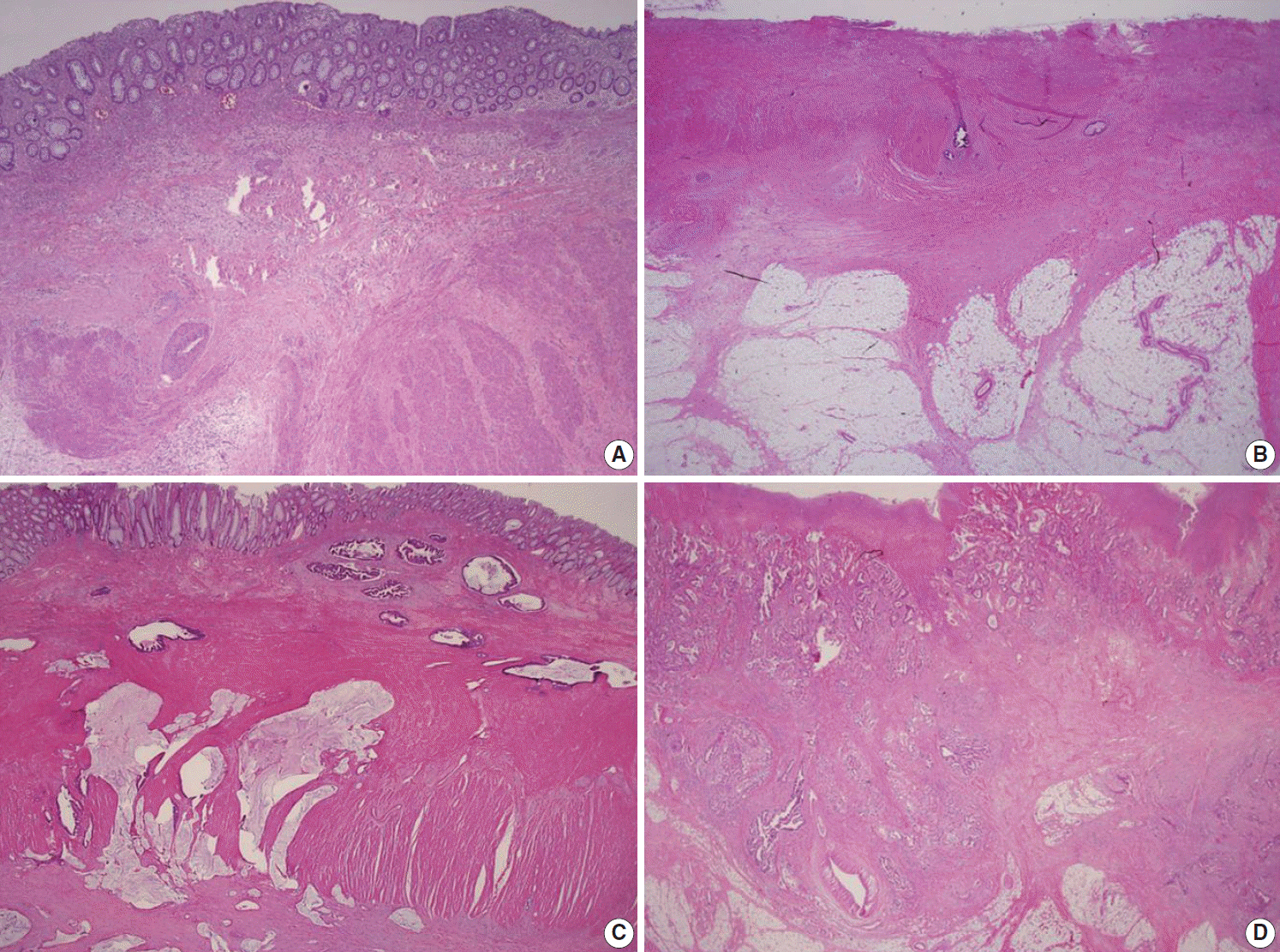
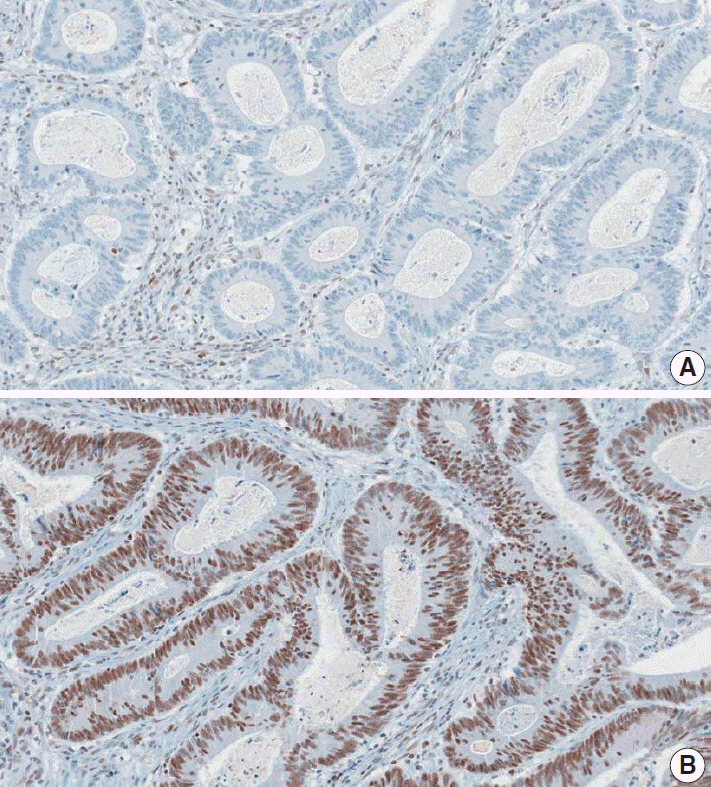
Figure
Cited by 2 articles
-
Polo-like kinase 4 as a potential predictive biomarker of chemoradioresistance in locally advanced rectal cancer
Hyunseung Oh, Soon Gu Kim, Sung Uk Bae, Sang Jun Byun, Shin Kim, Jae-Ho Lee, Ilseon Hwang, Sun Young Kwon, Hye Won Lee
J Pathol Transl Med. 2022;56(1):40-47. doi: 10.4132/jptm.2021.10.07.A standardized pathology report for gastric cancer: 2nd edition
Young Soo Park, Myeong-Cherl Kook, Baek-hui Kim, Hye Seung Lee, Dong-Wook Kang, Mi-Jin Gu, Ok Ran Shin, Younghee Choi, Wonae Lee, Hyunki Kim, In Hye Song, Kyoung-Mee Kim, Hee Sung Kim, Guhyun Kang, Do Youn Park, So-Young Jin, Joon Mee Kim, Yoon Jung Choi, Hee Kyung Chang, Soomin Ahn, Mee Soo Chang, Song-Hee Han, Yoonjin Kwak, An Na Seo, Sung Hak Lee, Mee-Yon Cho
J Pathol Transl Med. 2023;57(1):1-27. doi: 10.4132/jptm.2022.12.23.
Reference
-
1. Chang HJ, Park CK, Kim WH, et al. A standardized pathology report for colorectal cancer. Korean J Pathol. 2006; 40:193–203.2. Ministry of Health and Welfare. Annual report of cancer statistics in Korea in 2015. Sejong: Ministry of Health and Welfare;2017.3. Kakar S, Shi C, Berho ME, et al. Protocol for the examination of specimens from patients with primary carcinoma of the colon and rectum, version 4.0.0.0. Illinois: College of American Pathologists;2017.4. Amin MB, Edge S, Greene F, et al. AJCC cancer staging system. 8th ed. New York: Springer;2017.5. WHO Classification of Tumours Editorial Board. WHO classification of tumours: digestive system tumours. 5th ed. Geneva: World Health Organization;2019.6. Fritz AG, Percy C, Jack A, et al. International classification of diseases for oncology (ICD-O). 3rd ed. 1st rev. Lyon: International Agency for Research on Cancer;2013.7. Langman G, Loughrey M, Shepherd N, Quirke P. Association of Coloproctology of Great Britain & Ireland (ACPGBI): guidelines for the management of cancer of the colon, rectum and anus (2017)-pathology standards and datasets. Colorectal Dis. 2017; 19 Suppl 1:74–81.8. Nagtegaal I, Gaspar C, Marijnen C, Van De Velde C, Fodde R, Van Krieken H. Morphological changes in tumour type after radiotherapy are accompanied by changes in gene expression profile but not in clinical behaviour. J Pathol. 2004; 204:183–92.
Article9. Verdu M, Roman R, Calvo M, et al. Clinicopathological and molecular characterization of colorectal micropapillary carcinoma. Mod Pathol. 2011; 24:729–38.
Article10. Lee HJ, Eom DW, Kang GH, et al. Colorectal micropapillary carcinomas are associated with poor prognosis and enriched in markers of stem cells. Mod Pathol. 2013; 26:1123–31.
Article11. Nassar H. Carcinomas with micropapillary morphology: clinical significance and current concepts. Adv Anat Pathol. 2004; 11:297–303.12. Xu F, Xu J, Lou Z, et al. Micropapillary component in colorectal carcinoma is associated with lymph node metastasis in T1 and T2 Stages and decreased survival time in TNM stages I and II. Am J Surg Pathol. 2009; 33:1287–92.
Article13. Haupt B, Ro JY, Schwartz MR, Shen SS. Colorectal adenocarcinoma with micropapillary pattern and its association with lymph node metastasis. Mod Pathol. 2007; 20:729–33.
Article14. Jakubowska K, Guzinska-Ustymowicz K, Pryczynicz A. Invasive micropapillary component and its clinico-histopathological significance in patients with colorectal cancer. Oncol Lett. 2016; 12:1154–8.
Article15. Tuppurainen K, Makinen JM, Junttila O, et al. Morphology and microsatellite instability in sporadic serrated and non-serrated colorectal cancer. J Pathol. 2005; 207:285–94.
Article16. Patai AV, Molnár B, Tulassay Z, Sipos F. Serrated pathway: alternative route to colorectal cancer. World J Gastroenterol. 2013; 19:607–15.
Article17. Mäkinen MJ. Colorectal serrated adenocarcinoma. Histopathology. 2007; 50:131–50.
Article18. Barresi V, Reggiani Bonetti L, Ieni A, Domati F, Tuccari G. Prognostic significance of grading based on the counting of poorly differentiated clusters in colorectal mucinous adenocarcinoma. Hum Pathol. 2015; 46:1722–9.
Article19. Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO classification of tumours of the digestive system. 4th ed. Lyon: International Agency for Research on Cancer;2010.20. Kojima M, Shimazaki H, Iwaya K, et al. Intramucosal colorectal carcinoma with invasion of the lamina propria: a study by the Japanese Society for Cancer of the Colon and Rectum. Hum Pathol. 2017; 66:230–7.
Article21. Panarelli NC, Schreiner AM, Brandt SM, Shepherd NA, Yantiss RK. Histologic features and cytologic techniques that aid pathologic stage assessment of colonic adenocarcinoma. Am J Surg Pathol. 2013; 37:1252–8.
Article22. Uehara K, Nakanishi Y, Shimoda T, Taniguchi H, Akasu T, Moriya Y. Clinicopathological significance of microscopic abscess formation at the invasive margin of advanced low rectal cancer. Br J Surg. 2007; 94:239–43.
Article23. Smith KD, Tan D, Das P, et al. Clinical significance of acellular mucin in rectal adenocarcinoma patients with a pathologic complete response to preoperative chemoradiation. Ann Surg. 2010; 251:261–4.
Article24. Kang CM, Lim SB, Hong SM, et al. Prevalence and clinical significance of cellular and acellular mucin in patients with locally advanced mucinous rectal cancer who underwent preoperative chemoradiotherapy followed by radical surgery. Colorectal Dis. 2016; 18:O10–6.
Article25. Kitajima K, Fujimori T, Fujii S, et al. Correlations between lymph node metastasis and depth of submucosal invasion in submucosal invasive colorectal carcinoma: a Japanese collaborative study. J Gastroenterol. 2004; 39:534–43.
Article26. Bosch SL, Teerenstra S, de Wilt JH, Cunningham C, Nagtegaal ID. Predicting lymph node metastasis in pT1 colorectal cancer: a systematic review of risk factors providing rationale for therapy decisions. Endoscopy. 2013; 45:827–34.
Article27. Haggitt RC, Glotzbach RE, Soffer EE, Wruble LD. Prognostic factors in colorectal carcinomas arising in adenomas: implications for lesions removed by endoscopic polypectomy. Gastroenterology. 1985; 89:328–36.
Article28. Kikuchi R, Takano M, Takagi K, et al. Management of early invasive colorectal cancer: risk of recurrence and clinical guidelines. Dis Colon Rectum. 1995; 38:1286–95.29. Watanabe T, Muro K, Ajioka Y, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2016 for the treatment of colorectal cancer. Int J Clin Oncol. 2018; 23:1–34.
Article30. Betge J, Pollheimer MJ, Lindtner RA, et al. Intramural and extramural vascular invasion in colorectal cancer: prognostic significance and quality of pathology reporting. Cancer. 2012; 118:628–38.31. Kim WH, Park CK, Kim YB, et al. A standardized pathology report for gastric cancer. Korean J Pathol. 2005; 39:106–13.32. Liebig C, Ayala G, Wilks J, et al. Perineural invasion is an independent predictor of outcome in colorectal cancer. J Clin Oncol. 2009; 27:5131–7.
Article33. Liebig C, Ayala G, Wilks JA, Berger DH, Albo D. Perineural invasion in cancer: a review of the literature. Cancer. 2009; 115:3379–91.34. Gibson JA, Odze RD. Pathology of premalignant colorectal neoplasia. Dig Endosc. 2016; 28:312–23.
Article35. Rex DK, Ahnen DJ, Baron JA, et al. Serrated lesions of the colorectum: review and recommendations from an expert panel. Am J Gastroenterol. 2012; 107:1315–29.
Article36. Bateman AC, Shepherd NA. UK guidance for the pathological reporting of serrated lesions of the colorectum. J Clin Pathol. 2015; 68:585–91.
Article37. Liu C, Walker NI, Leggett BA, Whitehall VL, Bettington ML, Rosty C. Sessile serrated adenomas with dysplasia: morphological patterns and correlations with MLH1 immunohistochemistry. Mod Pathol. 2017; 30:1728–38.
Article38. Chetty R, Hafezi-Bakhtiari S, Serra S, Colling R, Wang LM. Traditional serrated adenomas (TSAs) admixed with other serrated (socalled precursor) polyps and conventional adenomas: a frequent occurrence. J Clin Pathol. 2015; 68:270–3.
Article39. Anwar MA, D'Souza F, Coulter R, Memon B, Khan IM, Memon MA. Outcome of acutely perforated colorectal cancers: experience of a single district general hospital. Surg Oncol. 2006; 15:91–6.
Article40. Mitrovic B, Schaeffer DF, Riddell RH, Kirsch R. Tumor budding in colorectal carcinoma: time to take notice. Mod Pathol. 2012; 25:1315–25.
Article41. Choi DH, Sohn DK, Chang HJ, Lim SB, Choi HS, Jeong SY. Indications for subsequent surgery after endoscopic resection of submucosally invasive colorectal carcinomas: a prospective cohort study. Dis Colon Rectum. 2009; 52:438–45.42. Ueno H, Mochizuki H, Hashiguchi Y, et al. Risk factors for an adverse outcome in early invasive colorectal carcinoma. Gastroenterology. 2004; 127:385–94.
Article43. Wang LM, Kevans D, Mulcahy H, et al. Tumor budding is a strong and reproducible prognostic marker in T3N0 colorectal cancer. Am J Surg Pathol. 2009; 33:134–41.
Article44. Giger OT, Comtesse SC, Lugli A, Zlobec I, Kurrer MO. Intra-tumoral budding in preoperative biopsy specimens predicts lymph node and distant metastasis in patients with colorectal cancer. Mod Pathol. 2012; 25:1048–53.
Article45. Rogers AC, Gibbons D, Hanly AM, et al. Prognostic significance of tumor budding in rectal cancer biopsies before neoadjuvant therapy. Mod Pathol. 2014; 27:156–62.
Article46. Lugli A, Kirsch R, Ajioka Y, et al. Recommendations for reporting tumor budding in colorectal cancer based on the International Tumor Budding Consensus Conference (ITBCC) 2016. Mod Pathol. 2017; 30:1299–311.
Article47. Koelzer VH, Zlobec I, Berger MD, et al. Tumor budding in colorectal cancer revisited: results of a multicenter interobserver study. Virchows Arch. 2015; 466:485–93.
Article48. Koelzer VH, Assarzadegan N, Dawson H, et al. Cytokeratinbased assessment of tumour budding in colorectal cancer: analysis in stage II patients and prospective diagnostic experience. J Pathol Clin Res. 2017; 3:171–8.
Article49. Jass JR, Love SB, Northover JM. A new prognostic classification of rectal cancer. Lancet. 1987; 1:1303–6.
Article50. Bosch SL, Nagtegaal ID. The importance of the pathologist’s role in assessment of the quality of the mesorectum. Curr Colorectal Cancer Rep. 2012; 8:90–8.
Article51. Aklilu M, Eng C. The current landscape of locally advanced rectal cancer. Nat Rev Clin Oncol. 2011; 8:649–59.
Article52. Bibeau F, Rullier A, Jourdan MF, et al. Locally advanced rectal cancer management: which role for the pathologist in 2011? Ann Pathol. 2011; 31:433–41.53. Dhadda AS, Dickinson P, Zaitoun AM, Gandhi N, Bessell EM. Prognostic importance of Mandard tumour regression grade following pre-operative chemo/radiotherapy for locally advanced rectal cancer. Eur J Cancer. 2011; 47:1138–45.
Article54. Mihaylova I, Parvanova V, Velikova C, Kurteva G, Ivanova D. Degree of tumor regression after preoperative chemo-radiotherapy in locally advanced rectal cancer-Preliminary results. Rep Pract Oncol Radiother. 2011; 16:237–42.
Article55. Vallböhmer D, Bollschweiler E, Brabender J, et al. Evaluation of histological regression grading systems in the neoadjuvant therapy of rectal cancer: do they have prognostic impact? Int J Colorectal Dis. 2012; 27:1295–301.56. Kim SH, Chang HJ, Kim DY, et al. What is the ideal tumor regression grading system in rectal cancer patients after preoperative chemoradiotherapy? Cancer Res Treat. 2016; 48:998–1009.
Article57. Mandard AM, Dalibard F, Mandard JC, et al. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma: clinicopathologic correlations. Cancer. 1994; 73:2680–6.
Article58. Dworak O, Keilholz L, Hoffmann A. Pathological features of rectal cancer after preoperative radiochemotherapy. Int J Colorectal Dis. 1997; 12:19–23.
Article59. Ryan R, Gibbons D, Hyland JM, et al. Pathological response following long-course neoadjuvant chemoradiotherapy for locally advanced rectal cancer. Histopathology. 2005; 47:141–6.
Article60. Krasinskas AM. EGFR Signaling in Colorectal Carcinoma. Patholog Res Int. 2011; 2011:932932.
Article61. Goldstein NS, Armin M. Epidermal growth factor receptor immunohistochemical reactivity in patients with American Joint Committee on Cancer Stage IV colon adenocarcinoma: implications for a standardized scoring system. Cancer. 2001; 92:1331–46.62. McKay JA, Murray LJ, Curran S, et al. Evaluation of the epidermal growth factor receptor (EGFR) in colorectal tumours and lymph node metastases. Eur J Cancer. 2002; 38:2258–64.
Article63. Spano JP, Fagard R, Soria JC, Rixe O, Khayat D, Milano G. Epidermal growth factor receptor signaling in colorectal cancer: preclinical data and therapeutic perspectives. Ann Oncol. 2005; 16:189–94.
Article64. Resnick MB, Routhier J, Konkin T, Sabo E, Pricolo VE. Epidermal growth factor receptor, c-MET, beta-catenin, and p53 expression as prognostic indicators in stage II colon cancer: a tissue microarray study. Clin Cancer Res. 2004; 10:3069–75.65. de Castro-Carpeno J, Belda-Iniesta C, Casado Saenz E, Hernandez Agudo E, Feliu Batlle J, Gonzalez Baron M. EGFR and colon cancer: a clinical view. Clin Transl Oncol. 2008; 10:6–13.
Article66. Saltz LB, Meropol NJ, Loehrer PJ Sr, Needle MN, Kopit J, Mayer RJ. Phase II trial of cetuximab in patients with refractory colorectal cancer that expresses the epidermal growth factor receptor. J Clin Oncol. 2004; 22:1201–8.
Article67. Vallböhmer D, Zhang W, Gordon M, et al. Molecular determinants of cetuximab efficacy. J Clin Oncol. 2005; 23:3536–44.
Article68. Ogino S, Meyerhardt JA, Cantor M, et al. Molecular alterations in tumors and response to combination chemotherapy with gefitinib for advanced colorectal cancer. Clin Cancer Res. 2005; 11:6650–6.
Article69. Geiersbach KB, Samowitz WS. Microsatellite instability and colorectal cancer. Arch Pathol Lab Med. 2011; 135:1269–77.
Article70. Pino MS, Chung DC. Microsatellite instability in the management of colorectal cancer. Expert Rev Gastroenterol Hepatol. 2011; 5:385–99.
Article71. Samowitz WS. Evaluation of colorectal cancers for Lynch syndrome: practical molecular diagnostics for surgical pathologists. Mod Pathol. 2015; 28 Suppl 1:S109–13.
Article72. Boland CR, Goel A. Microsatellite instability in colorectal cancer. Gastroenterology. 2010; 138:2073–87.
Article73. Guastadisegni C, Colafranceschi M, Ottini L, Dogliotti E. Microsatellite instability as a marker of prognosis and response to therapy: a meta-analysis of colorectal cancer survival data. Eur J Cancer. 2010; 46:2788–98.
Article74. Pritchard CC, Grady WM. Colorectal cancer molecular biology moves into clinical practice. Gut. 2011; 60:116–29.
Article75. Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015; 372:2509–20.
Article76. Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017; 357:409–13.77. Roxburgh CS, McMillan DC. The role of the in situ local inflammatory response in predicting recurrence and survival in patients with primary operable colorectal cancer. Cancer Treat Rev. 2012; 38:451–66.
Article78. Kirilovsky A, Marliot F, El Sissy C, Haicheur N, Galon J, Pages F. Rational bases for the use of the Immunoscore in routine clinical settings as a prognostic and predictive biomarker in cancer patients. Int Immunol. 2016; 28:373–82.
Article79. Mlecnik B, Bindea G, Angell HK, et al. Integrative analyses of colorectal cancer show immunoscore is a stronger predictor of patient survival than microsatellite instability. Immunity. 2016; 44:698–711.
Article80. Galon J, Mlecnik B, Bindea G, et al. Towards the introduction of the ‘Immunoscore’ in the classification of malignant tumours. J Pathol. 2014; 232:199–209.
Article81. Galon J, Pagès F, Marincola FM, et al. Cancer classification using the Immunoscore: a worldwide task force. J Transl Med. 2012; 10:205.82. Sepulveda AR, Hamilton SR, Allegra CJ, et al. Molecular biomarkers for the evaluation of colorectal cancer: guideline from the American Society for Clinical Pathology, College of American Pathologists, Association for Molecular Pathology, and the American Society of Clinical Oncology. J Clin Oncol. 2017; 35:1453–86.
Article83. Zaanan A, Shi Q, Taieb J, et al. Role of deficient DNA mismatch repair status in patients with stage III colon cancer treated with FOLFOX adjuvant chemotherapy: a pooled analysis from 2 randomized clinical trials. JAMA Oncol. 2018; 4:379–83.84. Sargent DJ, Marsoni S, Monges G, et al. Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil-based adjuvant therapy in colon cancer. J Clin Oncol. 2010; 28:3219–26.
Article85. Overman MJ, McDermott R, Leach JL, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol. 2017; 18:1182–91.
Article86. Umar A, Boland CR, Terdiman JP, et al. Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst. 2004; 96:261–8.87. Boland CR, Thibodeau SN, Hamilton SR, et al. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998; 58:5248–57.88. Adar T, Rodgers LH, Shannon KM, et al. A tailored approach to BRAF and MLH1 methylation testing in a universal screening program for Lynch syndrome. Mod Pathol. 2017; 30:440–7.
Article89. Buhard O, Cattaneo F, Wong YF, et al. Multipopulation analysis of polymorphisms in five mononucleotide repeats used to determine the microsatellite instability status of human tumors. J Clin Oncol. 2006; 24:241–51.
Article90. Goel A, Nagasaka T, Hamelin R, Boland CR. An optimized pentaplex PCR for detecting DNA mismatch repair-deficient colorectal cancers. PLoS One. 2010; 5:e9393.
Article91. Van Cutsem E, Cervantes A, Adam R, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016; 27:1386–422.92. Amado RG, Wolf M, Peeters M, et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol. 2008; 26:1626–34.93. Douillard JY, Oliner KS, Siena S, et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med. 2013; 369:1023–34.94. Rui Y, Wang C, Zhou Z, Zhong X, Yu Y. K-Ras mutation and prognosis of colorectal cancer: a meta-analysis. Hepatogastroenterology. 2015; 62:19–24.95. Phipps AI, Limburg PJ, Baron JA, et al. Association between molecular subtypes of colorectal cancer and patient survival. Gastroenterology. 2015; 148:77–87.
Article96. Sinicrope FA, Shi Q, Smyrk TC, et al. Molecular markers identify subtypes of stage III colon cancer associated with patient outcomes. Gastroenterology. 2015; 148:88–99.
Article97. Taieb J, Le Malicot K, Shi Q, et al. Prognostic value of BRAF and KRAS mutations in MSI and MSS stage III colon cancer. J Natl Cancer Inst. 2017; 109:djw272.98. Schirripa M, Bergamo F, Cremolini C, et al. BRAF and RAS mutations as prognostic factors in metastatic colorectal cancer patients undergoing liver resection. Br J Cancer. 2015; 112:1921–8.99. Wang L, Cunningham JM, Winters JL, et al. BRAF mutations in colon cancer are not likely attributable to defective DNA mismatch repair. Cancer Res. 2003; 63:5209–12.100. Loes IM, Immervoll H, Sorbye H, et al. Impact of KRAS, BRAF, PIK3CA, TP53 status and intraindividual mutation heterogeneity on outcome after liver resection for colorectal cancer metastases. Int J Cancer. 2016; 139:647–56.101. Di Nicolantonio F, Martini M, Molinari F, et al. Wild-type BRAF is required for response to panitumumab or cetuximab in metastatic colorectal cancer. J Clin Oncol. 2008; 26:5705–12.102. Pietrantonio F, Petrelli F, Coinu A, et al. Predictive role of BRAF mutations in patients with advanced colorectal cancer receiving cetuximab and panitumumab: a meta-analysis. Eur J Cancer. 2015; 51:587–94.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Standardized Pathology Report for Colorectal Cancer
- A Standardized Pathology Report for Gastric Cancer: 2nd Edition
- A standardized pathology report for gastric cancer: 2nd edition
- Differences in Overall Survival When Colorectal Cancer Patients are Stratified into New TNM Staging Strategy
- Changing trends in colorectal cancer in the Republic of Korea: contrast with Japan