Intest Res.
2020 Jan;18(1):69-78. 10.5217/ir.2019.00084.
5-Aminosalicylic acid intolerance is associated with a risk of adverse clinical outcomes and dysbiosis in patients with ulcerative colitis
- Affiliations
-
- 1Division of Gastroenterology and Hepatology, Department of Internal Medicine, Keio University School of Medicine, Tokyo, Japan
- 2Endoscopy Center, Edogawa Hospital, Tokyo, Japan
- 3Department of Gastroenterology and Hepatology, Saitama Medical Center, Saitama, Japan
- 4Department of Gastroenterology and Hepatology, Tokyo Saiseikai Central Hospital, Tokyo, Japan
- 5Department of Health Policy and Management, Keio University School of Medicine, Tokyo, Japan
- 6Laboratory for Microbiome Sciences, RIKEN Center for Integrative Medical Sciences, Kanagawa, Japan
- KMID: 2501368
- DOI: http://doi.org/10.5217/ir.2019.00084
Abstract
- Background/Aims
5-Aminosalicylic acid (ASA) causes intolerance reactions in some patients. This study was performed to examine the prognosis of patients with ulcerative colitis (UC) and 5-ASA intolerance, and to evaluate the potential interaction between 5-ASA intolerance and the intestinal microbiota.
Methods
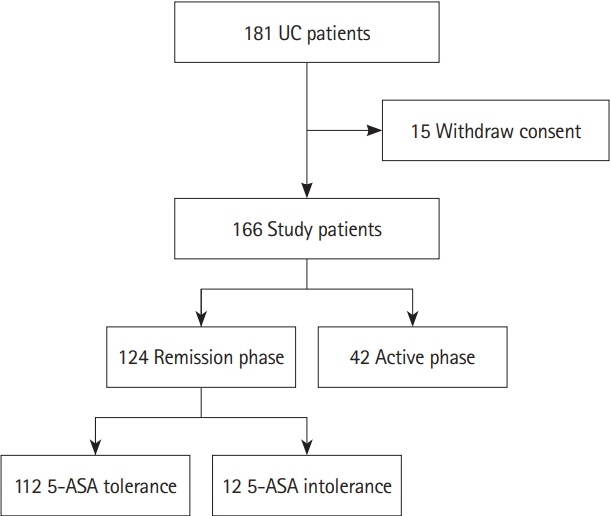
We performed a retrospective cohort study of patients with UC who visited participating hospitals. The primary endpoint was to compare the incidence of hospitalization within 12 months between the 5-ASA intolerance group and the 5-ASA tolerance group. The secondary endpoint was to compare the risk of adverse clinical outcomes after the start of biologics between the 2 groups. We also assessed the correlation between 5-ASA intolerance and microbial change in an independently recruited cohort of patients with UC.
Results
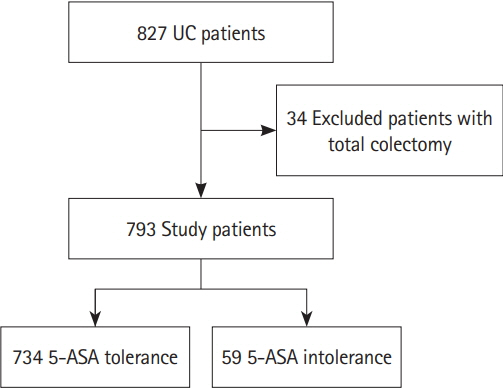
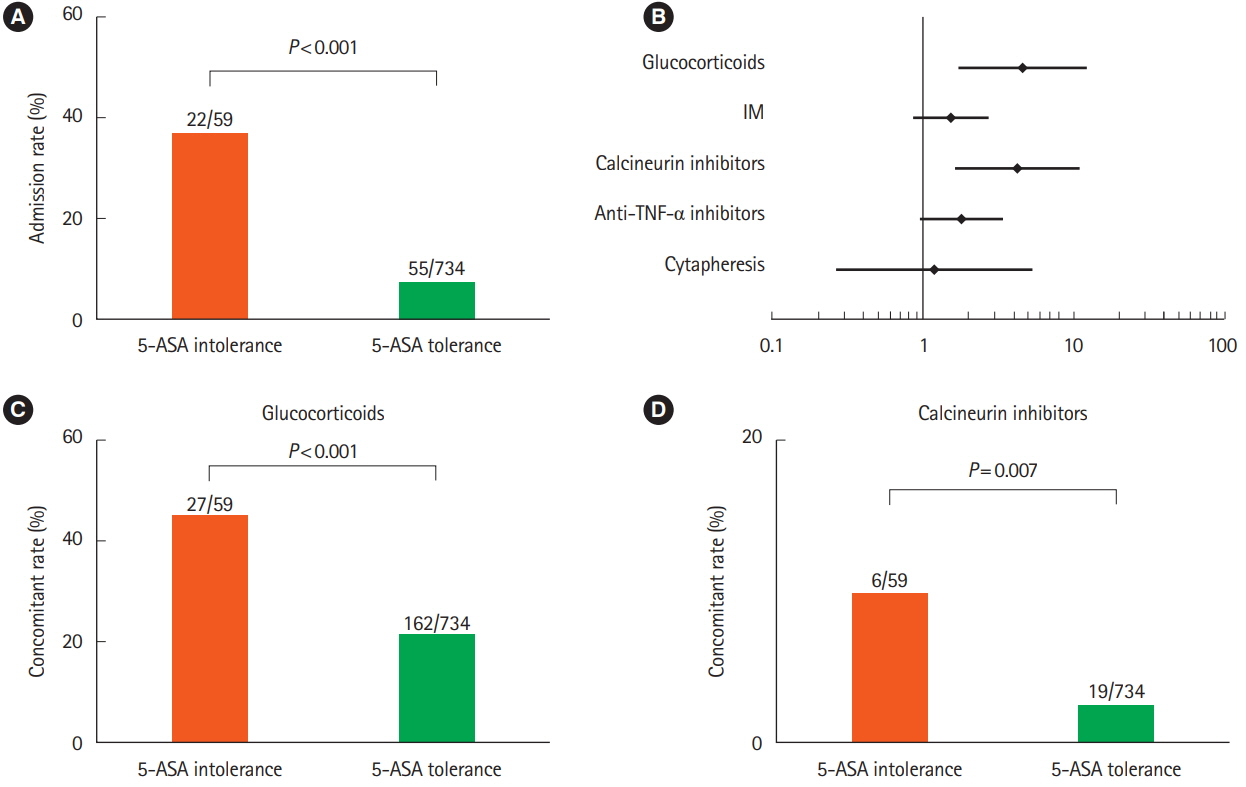
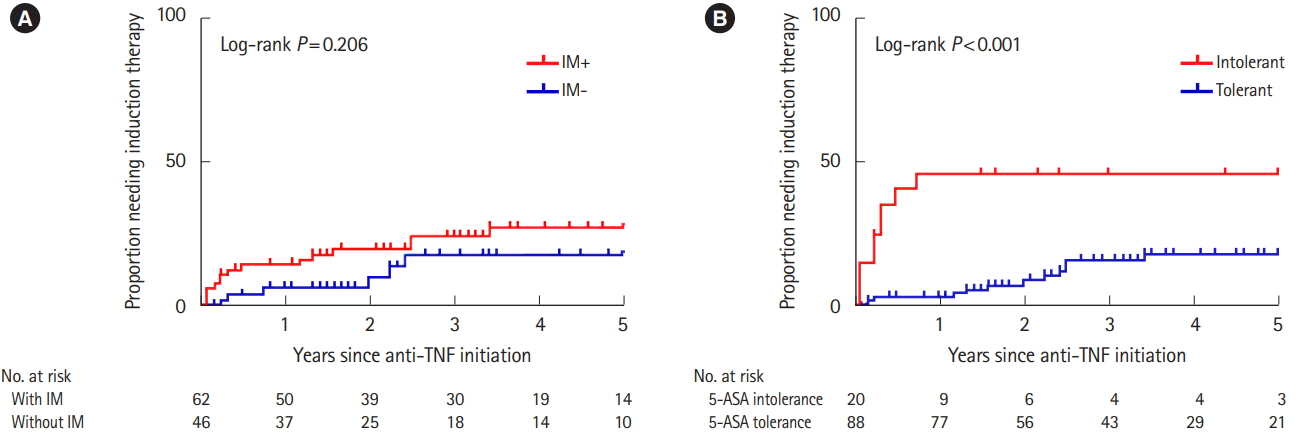
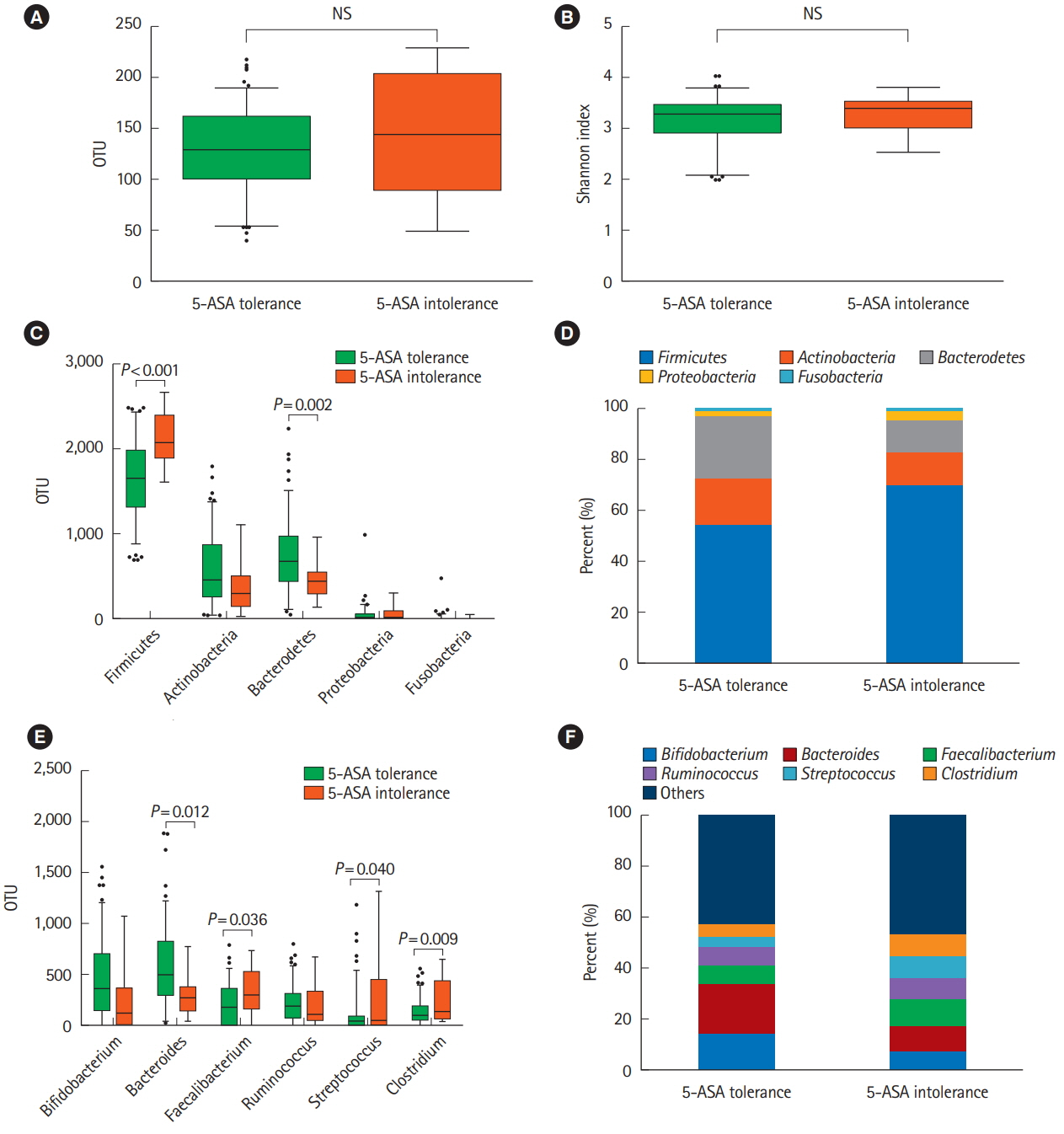
Of 793 patients, 59 (7.4%) were assigned to the 5-ASA intolerance group and 734 (92.5%) were assigned to the 5-ASA tolerance group. The admission rate and incidence of corticosteroid use were significantly higher in the intolerance than tolerance group (P< 0.001). In 108 patients undergoing treatment with anti-tumor necrosis factor biologics, 5-ASA intolerance increased the incidence of additional induction therapy after starting biologics (P< 0.001). The 5-ASA intolerance group had a greater abundance of bacteria in the genera Faecalibacterium, Streptococcus, and Clostridium than the 5-ASA tolerance group (P< 0.05).
Conclusions
In patients with UC, 5-ASA intolerance is associated with a risk of adverse clinical outcomes and dysbiosis. Bacterial therapeutic optimization of 5-ASA administration may be important for improving the prognosis of patients with UC.
Figure
Reference
-
1. Danese S, Fiocchi C. Ulcerative colitis. N Engl J Med. 2011; 365:1713–1725.2. Almario CV, Keller MS, Chen M, et al. Optimizing selection of biologics in inflammatory bowel disease: development of an online patient decision aid using conjoint analysis. Am J Gastroenterol. 2018; 113:58–71.3. White JR, Phillips F, Monaghan T, et al. Review article: novel oral-targeted therapies in inflammatory bowel disease. Aliment Pharmacol Ther. 2018; 47:1610–1622.4. Kaplan GG, Seow CH, Ghosh S, et al. Decreasing colectomy rates for ulcerative colitis: a population-based time trend study. Am J Gastroenterol. 2012; 107:1879–1887.5. Vester-Andersen MK, Prosberg MV, Jess T, et al. Disease course and surgery rates in inflammatory bowel disease: a population-based, 7-year follow-up study in the era of immunomodulating therapy. Am J Gastroenterol. 2014; 109:705–714.6. Pillai N, Dusheiko M, Burnand B, Pittet V. A systematic review of cost-effectiveness studies comparing conventional, biological and surgical interventions for inflammatory bowel disease. PLoS One. 2017; 12:e0185500.7. Ford AC, Achkar JP, Khan KJ, et al. Efficacy of 5-aminosalicylates in ulcerative colitis: systematic review and meta-analysis. Am J Gastroenterol. 2011; 106:601–616.8. Di Paolo MC, Paoluzi OA, Pica R, et al. Sulphasalazine and 5-aminosalicylic acid in long-term treatment of ulcerative colitis: report on tolerance and side-effects. Dig Liver Dis. 2001; 33:563–569.9. Ransford RA, Langman MJ. Sulphasalazine and mesalazine: serious adverse reactions re-evaluated on the basis of suspected adverse reaction reports to the Committee on Safety of Medicines. Gut. 2002; 51:536–539.10. Dignass A, Van Assche G, Lindsay JO, et al. The second European evidence-based consensus on the diagnosis and management of Crohn’s disease: current management. J Crohns Colitis. 2010; 4:28–62.11. Wang Y, Parker CE, Feagan BG, MacDonald JK. Oral 5-aminosalicylic acid for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev. 2016; (5):CD000544.12. Bernstein CN, Forbes JD. Gut microbiome in inflammatory bowel disease and other chronic immune-mediated inflammatory diseases. Inflamm Intest Dis. 2017; 2:116–123.
Article13. Zhang M, Sun K, Wu Y, Yang Y, Tso P, Wu Z. Interactions between intestinal microbiota and host immune response in inflammatory bowel disease. Front Immunol. 2017; 8:942.14. Shimizu H, Arai K, Tang J, Hosoi K, Funayama R. 5-Aminosalicylate intolerance causing exacerbation in pediatric ulcerative colitis. Pediatr Int. 2017; 59:583–587.
Article15. Nishijima S, Suda W, Oshima K, et al. The gut microbiome of healthy Japanese and its microbial and functional uniqueness. DNA Res. 2016; 23:125–133.16. Ungaro RC, Limketkai BN, Jensen CB, et al. Stopping 5-aminosalicylates in patients with ulcerative colitis starting biologic therapy does not increase the risk of adverse clinical outcomes: analysis of two nationwide population-based cohorts. Gut. 2019; 68:977–984.17. Bonovas S, Fiorino G, Lytras T, Nikolopoulos G, Peyrin-Biroulet L, Danese S. Systematic review with meta-analysis: use of 5-aminosalicylates and risk of colorectal neoplasia in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2017; 45:1179–1192.18. Harbord M, Eliakim R, Bettenworth D, et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. part 2: current management. J Crohns Colitis. 2017; 11:769–784.
Article19. Velayos FS, Terdiman JP, Walsh JM. Effect of 5-aminosalicylate use on colorectal cancer and dysplasia risk: a systematic review and metaanalysis of observational studies. Am J Gastroenterol. 2005; 100:1345–1353.
Article20. Zhao LN, Li JY, Yu T, Chen GC, Yuan YH, Chen QK. 5-Aminosalicylates reduce the risk of colorectal neoplasia in patients with ulcerative colitis: an updated meta-analysis. PLoS One. 2014; 9:e94208.
Article21. Tolia V. Sulfasalazine desensitization in children and adolescents with chronic inflammatory bowel disease. Am J Gastroenterol. 1992; 87:1029–1032.22. Heath JL, Heath RD, Tamboli C, et al. Mesalamine desensitization in a patient with treatment refractory ulcerative colitis and aspirin and nonsteroidal anti-inflammatory drug hypersensitivity. Ann Allergy Asthma Immunol. 2017; 118:518–520.
Article23. Panganiban CM, Pourang D, Samant SA. A novel 2-day desensitization protocol to oral mesalamine. J Allergy Clin Immunol Pract. 2018; 6:695–696.
Article24. Buurman DJ, De Monchy JG, Schellekens RC, van der Waaij LA, Kleibeuker JH, Dijkstra G. Ulcerative colitis patients with an inflammatory response upon mesalazine cannot be desensitized: a randomized study. Scand J Gastroenterol. 2015; 50:399–405.
Article25. Machiels K, Joossens M, Sabino J, et al. A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut. 2014; 63:1275–1283.
Article26. Noor SO, Ridgway K, Scovell L, et al. Ulcerative colitis and irritable bowel patients exhibit distinct abnormalities of the gut microbiota. BMC Gastroenterol. 2010; 10:134.
Article27. Qin N, Yang F, Li A, et al. Alterations of the human gut microbiome in liver cirrhosis. Nature. 2014; 513:59–64.
Article28. Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006; 444:1022–1023.29. Koliada A, Syzenko G, Moseiko V, et al. Association between body mass index and Firmicutes/Bacteroidetes ratio in an adult Ukrainian population. BMC Microbiol. 2017; 17:120.
Article30. Claesson MJ, Jeffery IB, Conde S, et al. Gut microbiota composition correlates with diet and health in the elderly. Nature. 2012; 488:178–184.
Article31. Hermiston ML, Gordon JI. Inflammatory bowel disease and adenomas in mice expressing a dominant negative N-cadherin. Science. 1995; 270:1203–1207.
Article32. Ley RE, Bäckhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A. 2005; 102:11070–11075.
Article33. Desai MS, Seekatz AM, Koropatkin NM, et al. A dietary fiberdeprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell. 2016; 167:1339–1353.
Article34. Damman CJ, Miller SI, Surawicz CM, Zisman TL. The microbiome and inflammatory bowel disease: is there a therapeutic role for fecal microbiota transplantation? Am J Gastroenterol. 2012; 107:1452–1459.
Article35. Derwa Y, Gracie DJ, Hamlin PJ, Ford AC. Systematic review with meta-analysis: the efficacy of probiotics in inflammatory bowel disease. Aliment Pharmacol Ther. 2017; 46:389–400.
Article36. Koretz RL. Probiotics in gastroenterology: how pro is the evidence in adults? Am J Gastroenterol. 2018; 113:1125–1136.
Article37. McIlroy J, Ianiro G, Mukhopadhya I, Hansen R, Hold GL. Review article: the gut microbiome in inflammatory bowel disease-avenues for microbial management. Aliment Pharmacol Ther. 2018; 47:26–42.
Article38. Halkjær SI, Christensen AH, Lo BZS, Browne PD, Günther S, Hansen LH, et al. Faecal microbiota transplantation alters gut microbiota in patients with irritable bowel syndrome: results from a randomised, double-blind placebo-controlled study. Gut. 2018; 67:2107–2115.
Article39. Johnsen PH, Hilpüsch F, Cavanagh JP, et al. Faecal microbiota transplantation versus placebo for moderate-to-severe irritable bowel syndrome: a double-blind, randomised, placebocontrolled, parallel-group, single-centre trial. Lancet Gastroenterol Hepatol. 2018; 3:17–24.
Article40. Kurokawa S, Kishimoto T, Mizuno S, et al. The effect of fecal microbiota transplantation on psychiatric symptoms among patients with irritable bowel syndrome, functional diarrhea and functional constipation: an open-label observational study. J Affect Disord. 2018; 235:506–512.
Article41. Mizuno S, Masaoka T, Naganuma M, et al. Bifidobacteriumrich fecal donor may be a positive predictor for successful fecal microbiota transplantation in patients with irritable bowel syndrome. Digestion. 2017; 96:29–38.
Article42. Dubin K, Callahan MK, Ren B, et al. Intestinal microbiome analyses identify melanoma patients at risk for checkpointblockade-induced colitis. Nat Commun. 2016; 7:10391.
Article43. Bajaj JS, Kassam Z, Fagan A, et al. Fecal microbiota transplant from a rational stool donor improves hepatic encephalopathy: a randomized clinical trial. Hepatology. 2017; 66:1727–1738.
Article44. Evrensel A, Ceylan ME. Fecal microbiota transplantation and its usage in neuropsychiatric disorders. Clin Psychopharmacol Neurosci. 2016; 14:231–237.
Article45. Bouter KE, van Raalte DH, Groen AK, Nieuwdorp M. Role of the gut microbiome in the pathogenesis of obesity and obesity-related metabolic dysfunction. Gastroenterology. 2017; 152:1671–1678.
Article46. Hampe CS, Roth CL. Probiotic strains and mechanistic insights for the treatment of type 2 diabetes. Endocrine. 2017; 58:207–227.
Article47. Wu H, Esteve E, Tremaroli V, et al. Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat Med. 2017; 23:850–858.
Article48. Zhu A, Chen J, Wu P, et al. Cationic polystyrene resolves nonalcoholic steatohepatitis, obesity, and metabolic disorders by promoting eubiosis of gut microbiota and decreasing endotoxemia. Diabetes. 2017; 66:2137–2143.
Article49. Costello SP, Soo W, Bryant RV, Jairath V, Hart AL, Andrews JM. Systematic review with meta-analysis: faecal microbiota transplantation for the induction of remission for active ulcerative colitis. Aliment Pharmacol Ther. 2017; 46:213–224.
Article50. Moayyedi P, Surette MG, Kim PT, et al. Fecal microbiota transplantation induces remission in patients with active ulcerative colitis in a randomized controlled trial. Gastroenterology. 2015; 149:102–109.
Article51. Narula N, Kassam Z, Yuan Y, et al. Systematic review and meta-analysis: fecal microbiota transplantation for treatment of active ulcerative colitis. Inflamm Bowel Dis. 2017; 23:1702–1709.52. Paramsothy S, Kamm MA, Kaakoush NO, et al. Multidonor intensive faecal microbiota transplantation for active ulcerative colitis: a randomised placebo-controlled trial. Lancet. 2017; 389:1218–1228.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- 5-aminosalicylic acid in the management of ulcerative colitis
- Perimyocarditis in a Patient with Ulcerative Colitis Treated with 5-Aminosalicylic Acid
- A Case of Acute Pancreatitis Caused by 5-aminosalicylic Acid Suppositories in a Patient with Ulcerative Colitis
- 5-Aminosalicylic Acid-induced Myocarditis in a Patient with Atypical Ulcerative Colitis
- Effect of acid-reducing agents on clinical relapse in ulcerative colitis with pH-dependent-released 5-aminosalicylic acid: a multicenter retrospective study in Japan