Ann Pediatr Endocrinol Metab.
2020 Mar;25(1):63-67. 10.6065/apem.2020.25.1.63.
Identification of a novel variant in the PHEX gene using targeted gene panel sequencing in a 24-month-old boy with hypophosphatemic rickets
- Affiliations
-
- 1Department of Pediatrics, Pusan National University Hospital, Pusan National University School of Medicine, Busan, Korea
- 2Green Cross Genome, Yongin, Korea
- KMID: 2501040
- DOI: http://doi.org/10.6065/apem.2020.25.1.63
Abstract
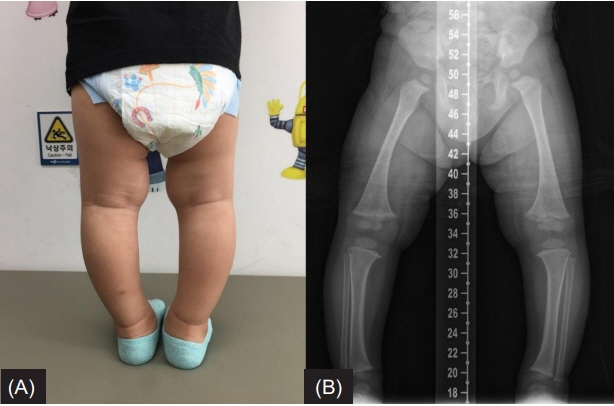
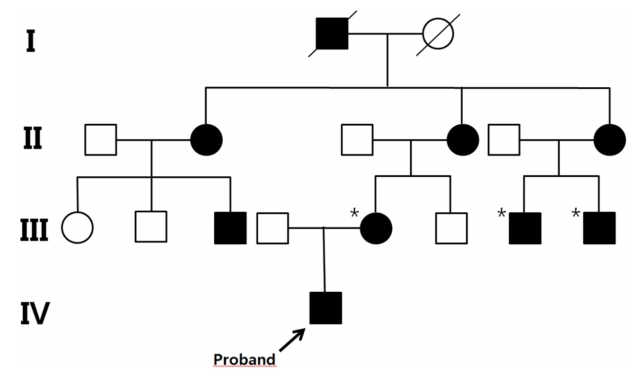
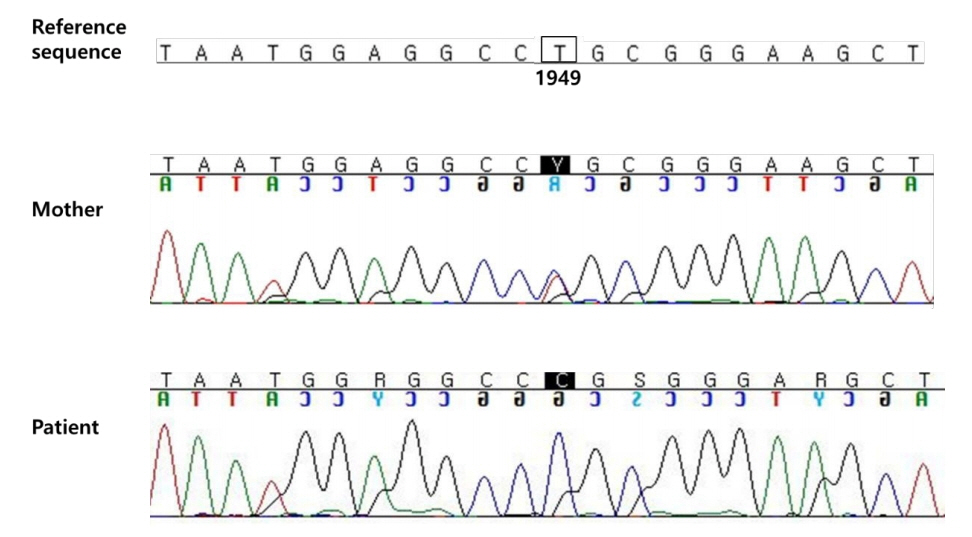
- Familial hypophosphatemic rickets (FHR) is a disorder characterized by phosphate wasting and hypophosphatemia due to defects in renal phosphate transport regulation. There are 4 known inherited forms of FHR that differ in their molecular causes. Very few studies have been conducted that focused on the molecular analysis of FHR in Koreans. Eighteen mutations of the PHEX gene have been identified to this date in Korea. Herein, we report the clinical case of a 24-month-old boy presenting with bowed legs and short stature. The biochemical profile showed hypophosphatemia with decreased tubular reabsorption of phosphate. Several family members were identified with short stature and genu varum. Therefore, he was diagnosed with FHR. To identify the molecular causes of FHR, we performed targeted gene panel sequencing and found a novel hemizygous missense variant, c.1949T>C (p.Leu650Pro), in the PHEX gene. This variant was also detected in the boy’s mother who exhibited genu varum and short stature.
Figure
Reference
-
References
1. Jan de Beur SM, Levine MA. Molecular pathogenesis of hypophosphatemic rickets. J Clin Endocrinol Metab. 2002; 87:2467–73.
Article2. A gene (PEX) with homologies to endopeptidases is mutated in patients with X-linked hypophosphatemic rickets. The HYP Consortium. Nat Genet. 1995; 11:130–6.3. ADHR Consortium. Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat Genet. 2000; 26:345–8.4. Lorenz-Depiereux B, Bastepe M, Benet-Pagès A, Amyere M, Wagenstaller J, Müller-Barth U, et al. DMP1 mutations in autosomal recessive hypophosphatemia implicate a bone matrix protein in the regulation of phosphate homeostasis. Nat Genet. 2006; 38:1248–50.
Article5. Lorenz-Depiereux B, Schnabel D, Tiosano D, Häusler G, Strom TM. Loss-of-function ENPP1 mutations cause both generalized arterial calcification of infancy and autosomalrecessive hypophosphatemic rickets. Am J Hum Genet. 2010; 86:267–72.
Article6. Strom TM, Jüppner H. PHEX, FGF23, DMP1 and beyond. Curr Opin Nephrol Hypertens. 2008; 17:357–62.
Article7. Cho HY, Lee BH, Kang JH, Ha IS, Cheong HI, Choi Y. A clinical and molecular genetic study of hypophosphatemic rickets in children. Pediatr Res. 2005; 58:329–33.
Article8. Song HR, Park JW, Cho DY, Yang JH, Yoon HR, Jung SC. PHEX gene mutations and genotype-phenotype analysis of Korean patients with hypophosphatemic rickets. J Korean Med Sci. 2007; 22:981–6.
Article9. Kim J, Yang KH, Nam JS, Choi JR, Song J, Chang M, et al. A novel PHEX mutation in a Korean patient with sporadic hypophosphatemic rickets. Ann Clin Lab Sci. 2009; 39:182–7.10. Cheon CK, Lee HS, Kim SY, Kwak MJ, Kim GH, Yoo HW. A novel de novo mutation within PHEX gene in a young girl with hypophosphatemic rickets and review of literature. Ann Pediatr Endocrinol Metab. 2014; 19:36–41.
Article11. Kang YE, Hong JH, Kim J, Joung KH, Kim HJ, Ku BJ, et al. A novel PHEX gene mutation in a patient with sporadic hypophosphatemic rickets. Endocrinol Metab (Seoul). 2014; 29:195–201.
Article12. Yang M, Kim J, Yang A, Jang J, Jeon TY, Cho SY, et al. A novel de novo mosaic mutation in PHEX in a Korean patient with hypophosphatemic rickets. Ann Pediatr Endocrinol Metab. 2018; 23:229–34.
Article13. Ng PC, Henikoff S. SIFT: Predicting amino acid changes that affect protein function. Nucleic Acids Res. 2003; 31:3812–4.
Article14. McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, et al. The genome analysis toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010; 20:1297–303.
Article15. Schwarz JM, Cooper DN, Schuelke M, Seelow D. MutationTaster2: mutation prediction for the deep sequencing age. Nat Methods. 2014; 11:361–2.
Article16. Holm IA, Nelson AE, Robinson BG, Mason RS, Marsh DJ, Cowell CT, et al. Mutational analysis and genotypephenotype correlation of the PHEX gene in X-linked hypophosphatemic rickets. J Clin Endocrinol Metab. 2001; 86:3889–99.
Article17. Sabbagh Y, Boileau G, DesGroseillers L, Tenenhouse HS. Disease-causing missense mutations in the PHEX gene interfere with membrane targeting of the recombinant protein. Hum Mol Genet. 2001; 10:1539–46.
Article18. Lee JY, Imel EA. The changing face of hypophosphatemic disorders in the FGF-23 era. Pediatr Endocrinol Rev. 2013; 10 Suppl 2:367–79.19. Linglart A, Biosse-Duplan M, Briot K, Chaussain C, Esterle L, Guillaume-Czitrom S, et al. Therapeutic management of hypophosphatemic rickets from infancy to adulthood. Endocr Connect. 2014; 3:R13–30.
Article20. Whyte MP, Carpenter TO, Gottesman GS, Mao M, Skrinar A, San Martin J, et al. Efficacy and safety of burosumab in children aged 1-4 years with X-linked hypophosphataemia: a multicentre, open-label, phase 2 trial. Lancet Diabetes Endocrinol. 2019; 7:189–99.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A novel de novo mosaic mutation in PHEX in a Korean patient with hypophosphatemic rickets
- A pathogenic PHEX variant (c.1483-1G>C) in a Korean patient with X-linked hypophosphatemic rickets
- A novel variant of PHEX in a Korean family with X-linked hypophosphatemic rickets
- PHEX Gene Mutations and Genotype-Phenotype Analysis of Korean Patients with Hypophosphatemic Rickets
- A Novel PHEX Gene Mutation in a Patient with Sporadic Hypophosphatemic Rickets