Int J Stem Cells.
2020 Mar;13(1):127-141. 10.15283/ijsc19111.
Neurogenin-1 Overexpression Increases the Therapeutic Effects of Mesenchymal Stem Cells through Enhanced Engraftment in an Ischemic Rat Brain
- Affiliations
-
- 1Department of Anatomy, Ajou University School of Medicine, Suwon, Korea
- 2Department of Biomedical Sciences, Ajou Graduate School, Suwon, Korea
- 3Research Center CelleBrain Ltd., Jeonju, Korea
- 4Department of Neurology, Ajou University School of Medicine, Suwon, Korea
- KMID: 2500572
- DOI: http://doi.org/10.15283/ijsc19111
Abstract
- Background and Objectives
Stem cell therapy is a promising strategy for treating neurological diseases but its effectiveness is influenced by the route of administration and the characteristics of the stem cells. We determined whether neural induction of mesenchymal stem cells (MSCs) was beneficial when the cells were delivered intra-arterially through the carotid artery.
Methods and Results
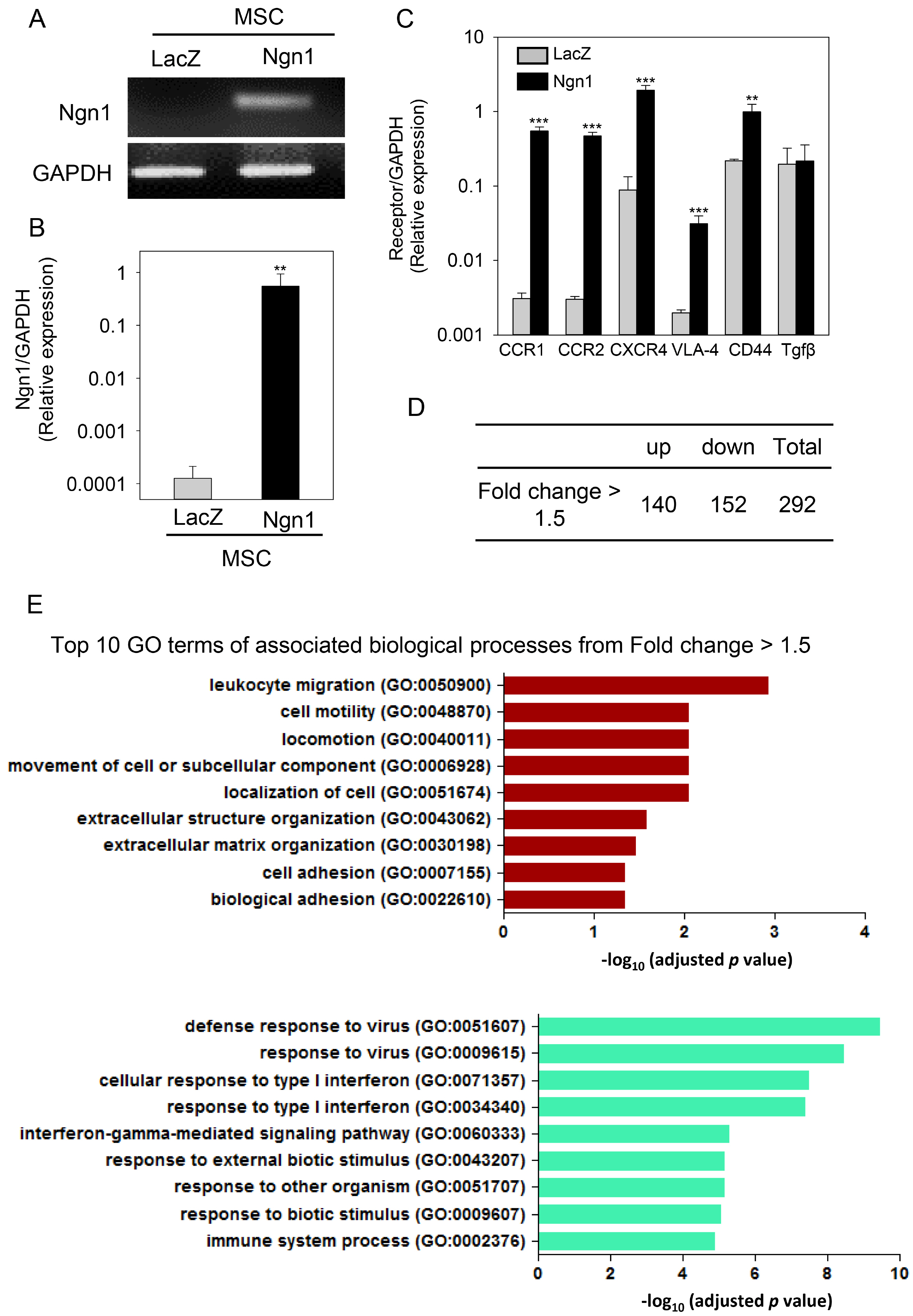
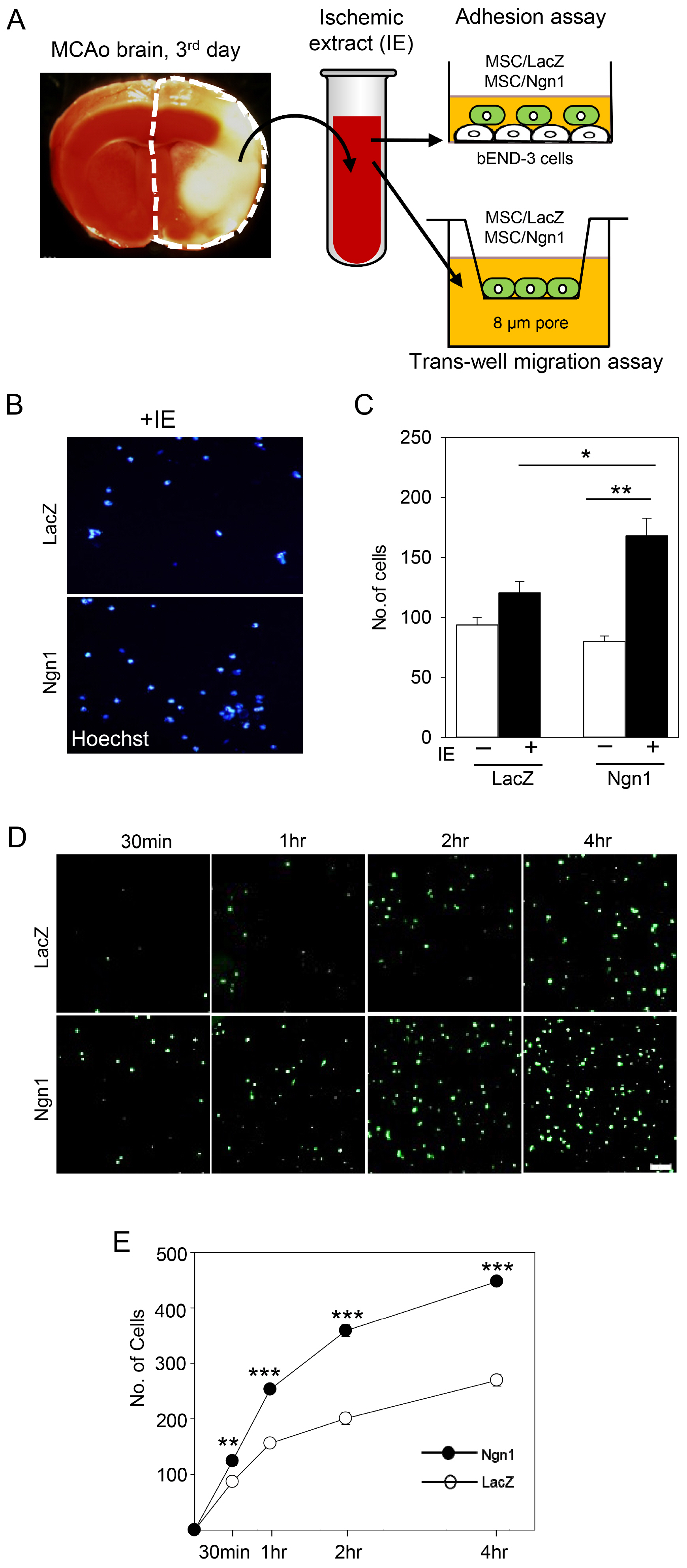
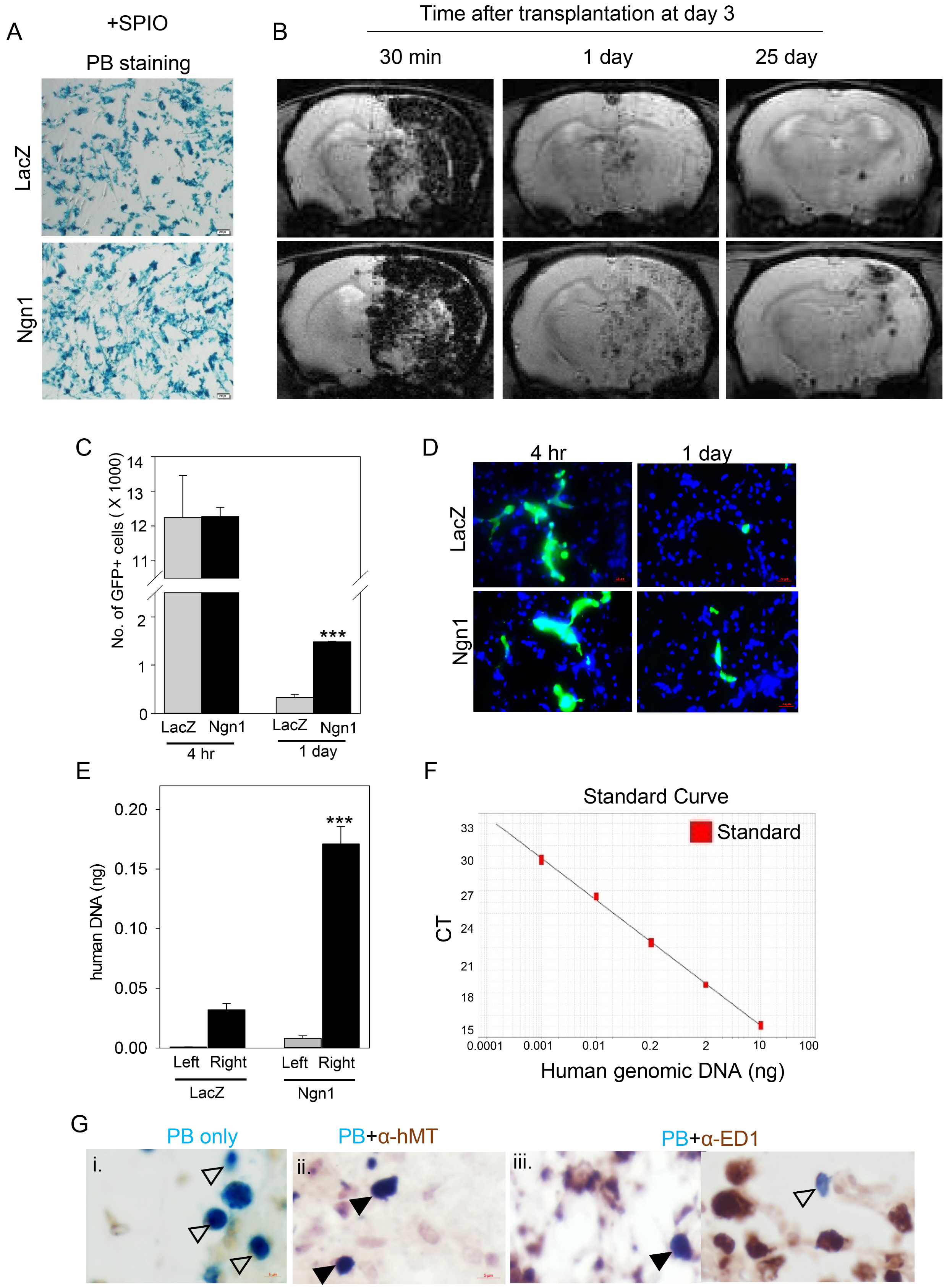
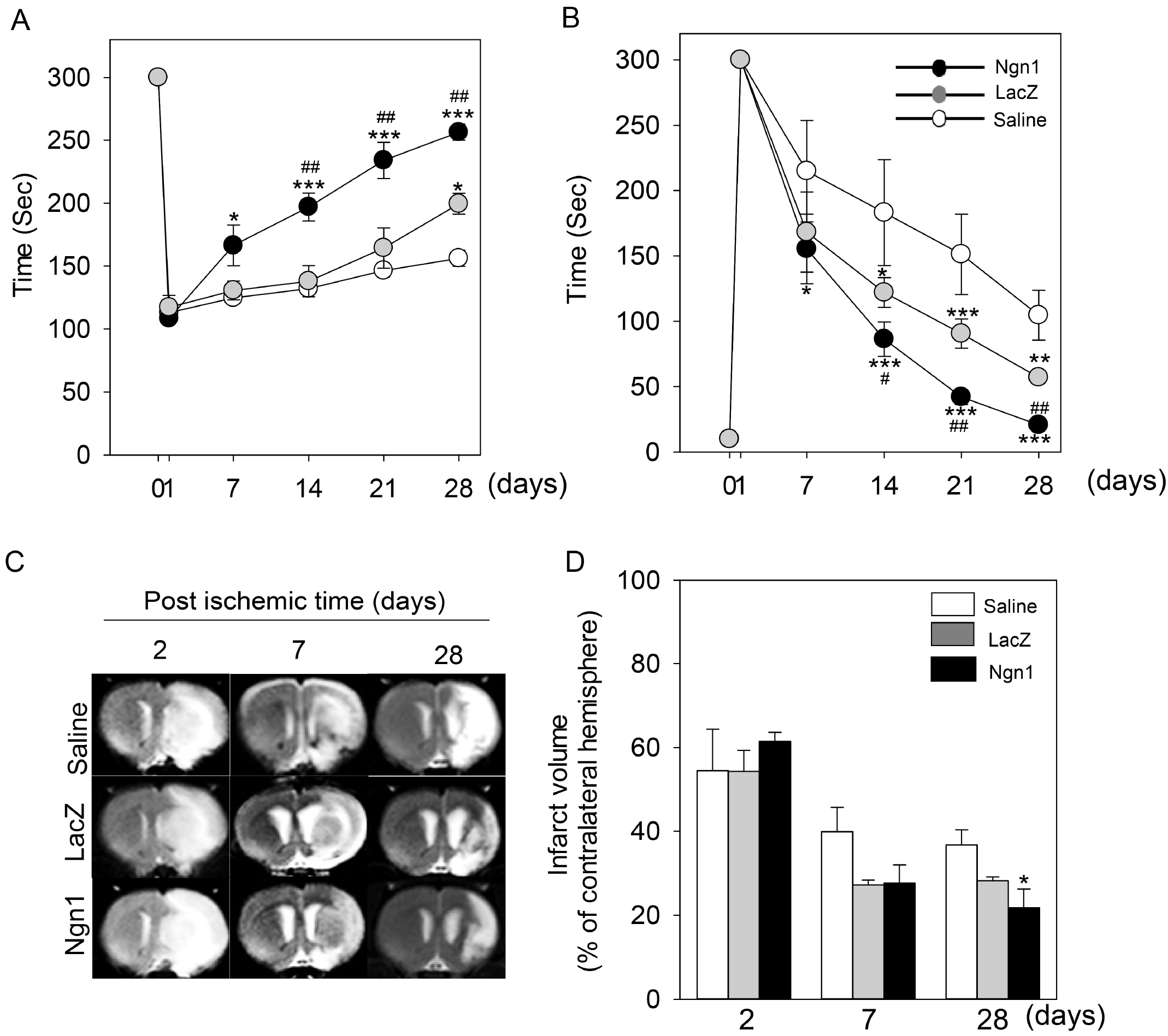
MSCs were neurally induced using a retroviral vector expressing the neurogenic transcription factor neurogenin-1 (Ngn1). The LacZ gene encoding bacterial β-galactosidase was used as a control. Ischemic stroke was induced by transluminal occlusion of the middle cerebral artery and 3 days later the MSCs were delivered intra- arterially through the internal carotid artery. Magnetic resonance imaging analysis indicated that compared to MSCs expressing LacZ (MSCs/LacZ), MSCs expressing Ngn1 (MSCs/Ngn1) exhibited increased recruitment to the ischemic region and populated this area for a longer duration. Immunohistochemical analysis indicated that compared to MSCs/LacZ, MSCs/Ngn1 more effectively alleviated neurological dysfunction by blocking secondary damage associated with neuronal cell death and brain inflammation. Microarray and real-time PCR analysis indicated that MSCs/Ngn1 exhibited increased expression of chemotactic cytokine receptors, adherence to endothelial cells, and migration ability.
Conclusions
Neural induction with Ngn1 increases the homing ability of MSCs, enhancing their engraftment efficiency in the ischemic rat brain. Intra-arterial delivery of neurally induced MSCs/Ngn1 3 days after ischemic injury blocks neuronal cell death and inflammation, and improves functional recovery. Thus, intra-arterial administration of stem cells with neural properties may be a novel therapy for the treatment of ischemic stroke.
Figure
Cited by 1 articles
-
Preclinical Study on Biodistribution of Mesenchymal Stem Cells after Local Transplantation into the Brain
Narayan Bashyal, Min Gyeong Kim, Jin-Hwa Jung, Rakshya Acharya, Young Jun Lee, Woo Sup Hwang, Jung-Mi Choi, Da-Young Chang, Sung-Soo Kim, Haeyoung Suh-Kim
Int J Stem Cells. 2023;16(4):415-424. doi: 10.15283/ijsc23062.
Reference
-
References
1. Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, de Ferranti SD, Ferguson JF, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Lutsey PL, Mackey JS, Matchar DB, Matsushita K, Mussolino ME, Nasir K, O'Flaherty M, Palaniappan LP, Pandey A, Pandey DK, Reeves MJ, Ritchey MD, Rodriguez CJ, Roth GA, Rosamond WD, Sampson UKA, Satou GM, Shah SH, Spartano NL, Tirschwell DL, Tsao CW, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P. American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. 2018; Heart disease and stroke statistics-2018 update: a report from the American Heart Association. Circulation. 137:e67–e492. DOI: 10.1161/CIR.0000000000000558. PMID: 29386200.
Article2. Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, Dávalos A, Majoie CB, van der Lugt A, de Miquel MA, Donnan GA, Roos YB, Bonafe A, Jahan R, Diener HC, van den Berg LA, Levy EI, Berkhemer OA, Pereira VM, Rempel J, Millán M, Davis SM, Roy D, Thornton J, Román LS, Ribó M, Beumer D, Stouch B, Brown S, Campbell BC, van Oostenbrugge RJ, Saver JL, Hill MD, Jovin TG. HERMES collaborators. 2016; Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 387:1723–1731. DOI: 10.1016/S0140-6736(16)00163-X. PMID: 26898852.
Article3. Marto JP, Lambrou D, Eskandari A, Nannoni S, Strambo D, Saliou G, Maeder P, Sirimarco G, Michel P. 2019; Associated factors and long-term prognosis of 24-hour worsening of arterial patency after ischemic stroke. Stroke. 50:2752–2760. DOI: 10.1161/STROKEAHA.119.025787. PMID: 31412758.
Article4. Yoon JS, Jo D, Lee HS, Yoo SW, Lee TY, Hwang WS, et al. 2018; Spatiotemporal protein atlas of cell death-related molecules in the rat MCAO stroke model. Exp Neurobiol. 27:287–98. DOI: 10.5607/en.2018.27.4.287. PMID: 30181691. PMCID: PMC6120968.
Article5. GBD 2016 Neurology Collaborators. 2019; Global, regional, and national burden of neurological disorders, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 18:459–480. DOI: 10.1016/S1474-4422(18)30499-X. PMID: 30879893. PMCID: PMC6459001.6. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. 1999; Multilineage potential of adult human mesenchymal stem cells. Science. 284:143–147. DOI: 10.1126/science.284.5411.143. PMID: 10102814.
Article7. Goldring CE, Duffy PA, Benvenisty N, Andrews PW, Ben-David U, Eakins R, French N, Hanley NA, Kelly L, Kitteringham NR, Kurth J, Ladenheim D, Laverty H, McBlane J, Narayanan G, Patel S, Reinhardt J, Rossi A, Sharpe M, Park BK. 2011; Assessing the safety of stem cell therapeutics. Cell Stem Cell. 8:618–628. DOI: 10.1016/j.stem.2011.05.012. PMID: 21624806.
Article8. Ben-David U, Benvenisty N. 2011; The tumorigenicity of human embryonic and induced pluripotent stem cells. Nat Rev Cancer. 11:268–277. DOI: 10.1038/nrc3034. PMID: 21390058.
Article9. Zomer HD, Vidane AS, Gonçalves NN, Ambrósio CE. 2015; Mesenchymal and induced pluripotent stem cells: general insights and clinical perspectives. Stem Cells Cloning. 8:125–134. DOI: 10.1016/S1525-0016(16)34271-X. PMID: 26451119. PMCID: PMC4592031.10. Bao X, Wei J, Feng M, Lu S, Li G, Dou W, Ma W, Ma S, An Y, Qin C, Zhao RC, Wang R. 2011; Transplantation of human bone marrow-derived mesenchymal stem cells promotes behavioral recovery and endogenous neurogenesis after cerebral ischemia in rats. Brain Res. 1367:103–113. DOI: 10.1016/j.brainres.2010.10.063. PMID: 20977892.
Article11. Kim SS, Yoo SW, Park TS, Ahn SC, Jeong HS, Kim JW, Chang DY, Cho KG, Kim SU, Huh Y, Lee JE, Lee SY, Lee YD, Suh-Kim H. 2008; Neural induction with neurogenin1 increases the therapeutic effects of mesenchymal stem cells in the ischemic brain. Stem Cells. 26:2217–2228. DOI: 10.1634/stemcells.2008-0108. PMID: 18617687.
Article12. Guan Y, Li X, Yu W, Liang Z, Huang M, Zhao R, Zhao C, Liu Y, Zou H, Hao Y, Chen Z. 2018; Intravenous transplantation of mesenchymal stem cells reduces the number of infiltrated Ly6C(+) cells but enhances the proportions positive for BDNF, TNF-1α, and IL-1β in the infarct cortices of dMCAO rats. Stem Cells Int. 2018:9207678. DOI: 10.1155/2018/9207678. PMID: 30405724. PMCID: PMC6189688.13. Chen J, Li Y, Katakowski M, Chen X, Wang L, Lu D, Lu M, Gautam SC, Chopp M. 2003; Intravenous bone marrow stromal cell therapy reduces apoptosis and promotes endogenous cell proliferation after stroke in female rat. J Neurosci Res. 73:778–786. DOI: 10.1002/jnr.10691. PMID: 12949903.
Article14. Sammali E, Alia C, Vegliante G, Colombo V, Giordano N, Pischiutta F, Boncoraglio GB, Barilani M, Lazzari L, Caleo M, De Simoni MG, Gaipa G, Citerio G, Zanier ER. 2017; Intravenous infusion of human bone marrow mesenchymal stromal cells promotes functional recovery and neuroplasticity after ischemic stroke in mice. Sci Rep. 7:6962. DOI: 10.1038/s41598-017-07274-w. PMID: 28761170. PMCID: PMC5537246.
Article15. Li Y, Chen J, Wang L, Lu M, Chopp M. 2001; Treatment of stroke in rat with intracarotid administration of marrow stromal cells. Neurology. 56:1666–1672. DOI: 10.1212/WNL.56.12.1666. PMID: 11425931.
Article16. Shen LH, Li Y, Chen J, Zhang J, Vanguri P, Borneman J, Chopp M. 2006; Intracarotid transplantation of bone marrow stromal cells increases axon-myelin remodeling after stroke. Neuroscience. 137:393–399. DOI: 10.1016/j.neuroscience.2005.08.092. PMID: 16298076.
Article17. Ruan GP, Han YB, Wang TH, Xing ZG, Zhu XB, Yao X, Ruan GH, Wang JX, Pang RQ, Cai XM, He J, Zhao J, Pan XH. 2013; Comparative study among three different methods of bone marrow mesenchymal stem cell transplantation following cerebral infarction in rats. Neurol Res. 35:212–220. DOI: 10.1179/1743132812Y.0000000152. PMID: 23452580.
Article18. Zhang HL, Xie XF, Xiong YQ, Liu SM, Hu GZ, Cao WF, Wu XM. 2018; Comparisons of the therapeutic effects of three different routes of bone marrow mesenchymal stem cell transplantation in cerebral ischemic rats. Brain Res. 1680:143–154. DOI: 10.1016/j.brainres.2017.12.017. PMID: 29274877.
Article19. Bang OY, Lee JS, Lee PH, Lee G. 2005; Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol. 57:874–882. DOI: 10.1002/ana.20501. PMID: 15929052.
Article20. Lee JS, Hong JM, Moon GJ, Lee PH, Ahn YH, Bang OY. STARTING collaborators. 2010; A long-term follow-up study of intravenous autologous mesenchymal stem cell transplantation in patients with ischemic stroke. Stem Cells. 28:1099–1106. DOI: 10.1002/stem.430. PMID: 20506226.
Article21. Honmou O, Houkin K, Matsunaga T, Niitsu Y, Ishiai S, Onodera R, Waxman SG, Kocsis JD. 2011; Intravenous administration of auto serum-expanded autologous mesenchymal stem cells in stroke. Brain. 134(Pt 6):1790–1807. DOI: 10.1093/brain/awr063. PMID: 21493695. PMCID: PMC3102237.
Article22. Jeong H, Yim HW, Cho YS, Kim YI, Jeong SN, Kim HB, et al. 2014; Efficacy and safety of stem cell therapies for patients with stroke: a systematic review and single arm meta-analysis. Int J Stem Cells. 7:63–9. DOI: 10.15283/ijsc.2014.7.2.63. PMID: 25473443. PMCID: PMC4249905.
Article23. Hess DC, Wechsler LR, Clark WM, Savitz SI, Ford GA, Chiu D, Yavagal DR, Uchino K, Liebeskind DS, Auchus AP, Sen S, Sila CA, Vest JD, Mays RW. 2017; Safety and efficacy of multipotent adult progenitor cells in acute ischaemic stroke (MASTERS): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol. 16:360–368. DOI: 10.1016/S1474-4422(17)30046-7. PMID: 28320635.
Article24. Wang S, Guo L, Ge J, Yu L, Cai T, Tian R, Jiang Y, Zhao RCh, Wu Y. 2015; Excess integrins cause lung entrapment of mesenchymal stem cells. Stem Cells. 33:3315–3326. DOI: 10.1002/stem.2087. PMID: 26148841.
Article25. Gholamrezanezhad A, Mirpour S, Bagheri M, Mohamadnejad M, Alimoghaddam K, Abdolahzadeh L, Saghari M, Malekzadeh R. 2011; In vivo tracking of 111In-oxine labeled mesenchymal stem cells following infusion in patients with advanced cirrhosis. Nucl Med Biol. 38:961–967. DOI: 10.1016/j.nucmedbio.2011.03.008. PMID: 21810549.
Article26. Shin DH, Kim GH, Lee JS, Joo IS, Suh-Kim H, Kim SS, Hong JM. 2016; Comparison of MSC-Neurogenin1 administration modality in MCAO rat model. Transl Neurosci. 7:164–172. DOI: 10.1515/tnsci-2016-0024. PMID: 28270935. PMCID: PMC5338457.
Article27. Kim SS, Choi JM, Kim JW, Ham DS, Ghil SH, Kim MK, Kim-Kwon Y, Hong SY, Ahn SC, Kim SU, Lee YD, Suh-Kim H. 2005; cAMP induces neuronal differentiation of mesenchymal stem cells via activation of extracellular signal-regulated kinase/MAPK. Neuroreport. 16:1357–1361. DOI: 10.1097/01.wnr.0000175243.12966.f5. PMID: 16056139.
Article28. Longa EZ, Weinstein PR, Carlson S, Cummins R. 1989; Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 20:84–91. DOI: 10.1161/01.STR.20.1.84. PMID: 2643202.
Article29. Park SY, Marasini S, Kim GH, Ku T, Choi C, Park MY, et al. 2014; A method for generating a mouse model of stroke: evaluation of parameters for blood flow, behavior, and survival [corrected]. Exp Neurobiol. 23:104–14. DOI: 10.5607/en.2014.23.1.104. PMID: 24737945. PMCID: PMC3984953.
Article30. Neumann-Haefelin T, Kastrup A, de Crespigny A, Yenari MA, Ringer T, Sun GH, Moseley ME. 2000; Serial MRI after transient focal cerebral ischemia in rats: dynamics of tissue injury, blood-brain barrier damage, and edema formation. Stroke. 31:1965–1972. discussion 1972-1973. DOI: 10.1161/01.STR.31.8.1965. PMID: 10926965.
Article31. Arbab AS, Yocum GT, Rad AM, Khakoo AY, Fellowes V, Read EJ, Frank JA. 2005; Labeling of cells with ferumoxides-protamine sulfate complexes does not inhibit function or differentiation capacity of hematopoietic or mesenchymal stem cells. NMR Biomed. 18:553–559. DOI: 10.1002/nbm.991. PMID: 16229060.
Article32. Chang DJ, Moon H, Lee YH, Lee N, Lee HJ, Jeon I, et al. 2012; In vivo tracking of human neural stem cells following transplantation into a rodent model of ischemic stroke. Int J Stem Cells. 5:79–83. DOI: 10.15283/ijsc.2012.5.1.79. PMID: 24298359. PMCID: PMC3840979.
Article33. Kurtz A. 2008; Mesenchymal stem cell delivery routes and fate. Int J Stem Cells. 1:1–7. DOI: 10.15283/ijsc.2008.1.1.1. PMID: 24855503. PMCID: PMC4021770.
Article34. Jiang L, Newman M, Saporta S, Chen N, Sanberg C, Sanberg PR, Willing AE. 2008; MIP-1alpha and MCP-1 Induce migration of human umbilical cord blood cells in models of stroke. Curr Neurovasc Res. 5:118–124. DOI: 10.2174/156720208784310259. PMID: 18473828.
Article35. Yamagami S, Tamura M, Hayashi M, Endo N, Tanabe H, Katsuura Y, Komoriya K. 1999; Differential production of MCP-1 and cytokine-induced neutrophil chemoattractant in the ischemic brain after transient focal ischemia in rats. J Leukoc Biol. 65:744–749. DOI: 10.1002/jlb.65.6.744. PMID: 10380894.
Article36. Hill WD, Hess DC, Martin-Studdard A, Carothers JJ, Zheng J, Hale D, Maeda M, Fagan SC, Carroll JE, Conway SJ. 2004; SDF-1 (CXCL12) is upregulated in the ischemic penumbra following stroke: association with bone marrow cell homing to injury. J Neuropathol Exp Neurol. 63:84–96. DOI: 10.1093/jnen/63.1.84. PMID: 14748564.
Article37. Zhong Y, Tang Z, Xu R, Lin N, Deng M, Fang H, Lin J, Zhu K, Liu Y, Kang Z. 2013; Effect of transplantation route on stem cell migration to fibrotic liver of rats via cellular magnetic resonance imaging. Cytotherapy. 15:1266–1274. DOI: 10.1016/j.jcyt.2013.05.023. PMID: 23993301.
Article38. Janowski M, Lyczek A, Engels C, Xu J, Lukomska B, Bulte JW, Walczak P. 2013; Cell size and velocity of injection are major determinants of the safety of intracarotid stem cell transplantation. J Cereb Blood Flow Metab. 33:921–927. DOI: 10.1038/jcbfm.2013.32. PMID: 23486296. PMCID: PMC3677113.
Article39. Yoo SW, Chang DY, Lee HS, Kim GH, Park JS, Ryu BY, Joe EH, Lee YD, Kim SS, Suh-Kim H. 2013; Immune following suppression mesenchymal stem cell transplantation in the ischemic brain is mediated by TGF-β. Neurobiol Dis. 58:249–257. DOI: 10.1016/j.nbd.2013.06.001. PMID: 23759293.
Article40. Steinberg GK, Kondziolka D, Wechsler LR, Lunsford LD, Coburn ML, Billigen JB, Kim AS, Johnson JN, Bates D, King B, Case C, McGrogan M, Yankee EW, Schwartz NE. 2016; Clinical outcomes of transplanted modified bone marrow-derived mesenchymal stem cells in stroke: a phase 1/2a study. Stroke. 47:1817–1824. DOI: 10.1161/STROKEAHA.116.012995. PMID: 27256670. PMCID: PMC5828512.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Trials of Adult Stem Cell Therapy in Patients with Ischemic Stroke
- Neural Stem Cells and Ischemic Brain
- Therapeutic Potential of Human Mesenchymal Stem Cells for Treating Ischemic Limb Diseases
- Combination Cell Therapy with Mesenchymal Stem Cells and Neural Stem Cells for Brain Stroke in Rats
- Transplantation of neural stem cells: cellular & gene therapy for hypoxic-ischemic brain injury