Anesth Pain Med.
2020 Jan;15(1):19-27. 10.17085/apm.2020.15.1.19.
Bile duct ligation of C57BL/6 mice as a model of hepatic encephalopathy
- Affiliations
-
- 1Department of Anesthesiology and Pain Medicine, Seoul, Korea
- 2Anesthesia and Pain Research Institute, Yonsei University College of Medicine, Seoul, Korea
- KMID: 2500450
- DOI: http://doi.org/10.17085/apm.2020.15.1.19
Abstract
- Background
Bile duct ligation (BDL) has been used for experimental research on hepatic encephalopathy (HE) caused by chronic liver disease. However, little research has been done on a BDL model in C57BL/6 mouse. Therefore, we evaluated the suitability of a BDL model in C57BL/6 mouse for the study of HE and determined which behavioral tests are appropriate for the identification of HE in this model.
Methods
Twelve to fourteen-week-old male C57BL/6 mice were randomly assigned to either sham group or BDL group. Histological changes in liver were confirmed by hematoxylin/ eosin and Masson’s trichrome staining. Liver function alterations were detected by alanine aminotransferase (ALT) and ammonia levels. To identify behavioral changes, open field, elevated plus maze, novel object recognition, and passive avoidance tests were performed.
Results
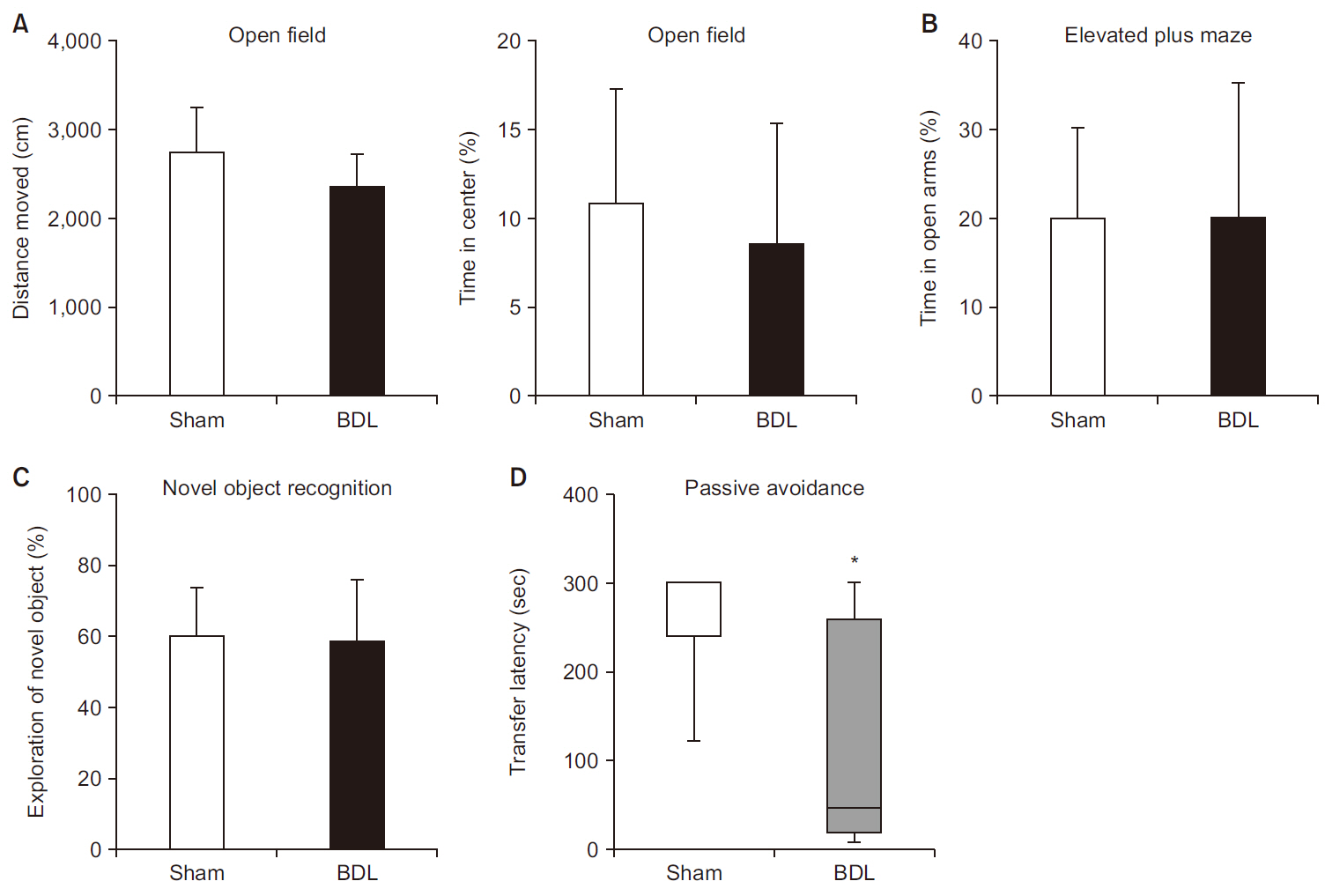
Inflammatory liver injury and fibrosis were observed 14 days after BDL. ALT and ammonia levels were significantly higher in BDL group than in sham group. There were no differences in general locomotor activity or anxiety between the groups. No difference was observed between these two groups in the novel object recognition test, but BDL group showed significant learning/memory impairment in the passive avoidance test compared to sham group.
Conclusions
Fourteen days of BDL in 12–14-week-old male C57BL/6 mice is a clinically relevant model for HE, as these mice have liver fibrosis with impaired liver function, hyperammonemia, and learning/memory impairment. Passive avoidance can be used as the major behavioral test in this model of HE.
Keyword
Figure
Reference
-
Vilstrup H., Amodio P., Bajaj J., Cordoba J., Ferenci P., Mullen KD, et al. 2014. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 60:715–35. DOI: 10.1002/hep.27210. PMID: 25042402.Basu PP., Shah NJ. 2015. Clinical and neurologic manifestation of minimal hepatic encephalopathy and overt hepatic encephalopathy. Clin Liver Dis. 19:461–72. DOI: 10.1016/j.cld.2015.05.003. PMID: 26195201.Butterworth RF., Norenberg MD., Felipo V., Ferenci P., Albrecht J., Blei AT. 2009. Experimental models of hepatic encephalopathy: ISHEN guidelines. Liver Int. 29:783–8. DOI: 10.1111/j.1478-3231.2009.02034.x. PMID: 19638106.McMillin M., Frampton G., Thompson M., Galindo C., Standeford H., Whittington E, et al. 2014. Neuronal CCL2 is upregulated during hepatic encephalopathy and contributes to microglia activation and neurological decline. J Neuroinflammation. 11:121. DOI: 10.1186/1742-2094-11-121. PMID: 25012628. PMCID: PMC4128607.Chastre A., Bélanger M., Nguyen BN., Butterworth RF. 2014. Lipopolysaccharide precipitates hepatic encephalopathy and increases blood-brain barrier permeability in mice with acute liver failure. Liver Int. 34:353–61. DOI: 10.1111/liv.12252. PMID: 23910048.Kerfoot SM., D'Mello C., Nguyen H., Ajuebor MN., Kubes P., Le T, et al. 2006. TNF-alpha-secreting monocytes are recruited into the brain of cholestatic mice. Hepatology. 43:154–62. DOI: 10.1002/hep.21003. PMID: 16374849.D'Mello C., Le T., Swain MG. 2009. Cerebral microglia recruit monocytes into the brain in response to tumor necrosis factoralpha signaling during peripheral organ inflammation. J Neurosci. 29:2089–102. DOI: 10.1523/JNEUROSCI.3567-08.2009. PMID: 19228962. PMCID: PMC6666330.Magen I., Avraham Y., Ackerman Z., Vorobiev L., Mechoulam R., Berry EM. 2009. Cannabidiol ameliorates cognitive and motor impairments in mice with bile duct ligation. J Hepatol. 51:528–34. DOI: 10.1016/j.jhep.2009.04.021. PMID: 19596476.Magen I., Avraham Y., Ackerman Z., Vorobiev L., Mechoulam R., Berry EM. 2010. Cannabidiol ameliorates cognitive and motor impairments in bile-duct ligated mice via 5-HT1A receptor activation. Br J Pharmacol. 159:950–7. DOI: 10.1111/j.1476-5381.2009.00589.x. PMID: 20128798. PMCID: PMC2829220.Zarrindast MR., Hoseindoost S., Nasehi M. 2012. Possible interaction between opioidergic and cholinergic systems of CA1 in cholestasis-induced amnesia in mice. Behav Brain Res. 228:116–24. DOI: 10.1016/j.bbr.2011.11.039. PMID: 22155612.Nasehi M., Mafi F., Ebrahimi-Ghiri M., Zarrindast MR. 2016. Function of opioidergic and dopaminergic antagonists on both spatial and object novelty detection deficits induced in rodent model of hepatic encephalopathy. Behav Brain Res. 313:58–66. DOI: 10.1016/j.bbr.2016.07.011. PMID: 27401106.Starkel P., Leclercq IA. 2011. Animal models for the study of hepatic fibrosis. Best Pract Res Clin Gastroenterol. 25:319–33. DOI: 10.1016/j.bpg.2011.02.004. PMID: 21497748.Tag CG., Weiskirchen S., Hittatiya K., Tacke F., Tolba RH., Weiskirchen R. 2015. Induction of experimental obstructive cholestasis in mice. Lab Anim. 49(1 Suppl):70–80. DOI: 10.1177/0023677214567748. PMID: 25835740.Tag CG., Sauer-Lehnen S., Weiskirchen S., Borkham-Kamphorst E., Tolba RH., Tacke F, et al. 2015. Bile duct ligation in mice: induction of inflammatory liver injury and fibrosis by obstructive cholestasis. J Vis Exp. (96):e52438. DOI: 10.3791/52438. PMID: 25741630. PMCID: PMC4354634.Rogers AB. 2018. Stress of strains: inbred mice in liver research. Gene Expr. 19:61–7. DOI: 10.3727/105221618X15337408678723. PMID: 30092856. PMCID: PMC6290319.Cheon SY., Kim JM., Kam EH., Ho CC., Kim EJ., Chung S, et al. 2017. Cell-penetrating interactomic inhibition of nuclear factor-kappa B in a mouse model of postoperative cognitive dysfunction. Sci Rep. 7:13482. DOI: 10.1038/s41598-017-14027-2. PMID: 29044209. PMCID: PMC5647420.Wolf A., Bauer B., Abner EL., Ashkenazy-Frolinger T., Hartz AM. 2016. A comprehensive behavioral test battery to assess learning and memory in 129S6/Tg2576 mice. PLoS One. 11:e0147733. DOI: 10.1371/journal.pone.0147733. PMID: 26808326. PMCID: PMC4726499.Farjam M., Dehdab P., Abbassnia F., Mehrabani D., Tanideh N., Pakbaz S, et al. 2012. Thioacetamide-induced acute hepatic encephalopathy in rat: behavioral, biochemical and histological changes. Iran Red Crescent Med J. 14:164–70. PMID: 22737573. PMCID: 3372030.Ilic S., Drmic D., Zarkovic K., Kolenc D., Coric M., Brcic L, et al. 2010. High hepatotoxic dose of paracetamol produces generalized convulsions and brain damage in rats. A counteraction with the stable gastric pentadecapeptide BPC 157 (PL 14736). J Physiol Pharmacol. 61:241–50. DOI: 10.1016/S0016-5085(09)61912-0. PMID: 20436226.Pacheco GS., Panatto JP., Fagundes DA., Scaini G., Bassani C., Jeremias IC, et al. 2009. Brain creatine kinase activity is inhibited after hepatic failure induced by carbon tetrachloride or acetaminophen. Metab Brain Dis. 24:383–94. DOI: 10.1007/s11011-009-9143-8. PMID: 19688255.Lee SS., Byoun YS., Jeong SH., Kim YM., Gil H., Min BY, et al. 2012. Type and cause of liver disease in Korea: single-center experience, 2005-2010. Clin Mol Hepatol. 18:309–15. DOI: 10.3350/cmh.2012.18.3.309. PMID: 23091812. PMCID: PMC3467435.Jackson SJ., Andrews N., Ball D., Bellantuono I., Gray J., Hachoumi L, et al. 2017. Does age matter? The impact of rodent age on study outcomes. Lab Anim. 51:160–9. DOI: 10.1177/0023677216653984. PMID: 27307423. PMCID: PMC5367550.Sousa N., Almeida OF., Wotjak CT. 2006. A hitchhiker's guide to behavioral analysis in laboratory rodents. Genes Brain Behav. 5(Suppl 2):5–24. DOI: 10.1111/j.1601-183X.2006.00228.x. PMID: 16681797.Kim JS., Yang MY., Son YH., Kim SH., Kim JC., Kim SJ, et al. 2008. Strain-dependent differences of locomotor activity and hippocampus-dependent learning and memory in mice. Toxicol Res. 24:183–8. DOI: 10.5487/TR.2008.24.3.183. PMID: 10837506. PMCID: 311331.Lueptow LM. 2017. Novel object recognition test for the investigation of learning and memory in mice. J Vis Exp. (126):e55718. DOI: 10.3791/55718. PMID: 28892027. PMCID: PMC5614391.Jayakumar AR., Norenberg MD. 2018. Hyperammonemia in hepatic encephalopathy. J Clin Exp Hepatol. 8:272–80. DOI: 10.1016/j.jceh.2018.06.007. PMID: 30302044. PMCID: PMC6175739.Gentile S., Guarino G., Romano M., Alagia IA., Fierro M., Annunziata S, et al. 2005. A randomized controlled trial of acarbose in hepatic encephalopathy. Clin Gastroenterol Hepatol. 3:184–91. DOI: 10.1016/S1542-3565(04)00667-6. PMID: 15704053.Rahimi RS., Rockey DC. 2016. Hepatic encephalopathy: pharmacological therapies targeting ammonia. Semin Liver Dis. 36:48–55. DOI: 10.1055/s-0036-1571298. PMID: 26870932.Soto-Gutiérrez A., Kobayashi N., Rivas-Carrillo JD., Navarro-Alvarez N., Zhao D., Okitsu T, et al. 2006. Reversal of mouse hepatic failure using an implanted liver-assist device containing ES cell-derived hepatocytes. Nat Biotechnol. 24:1412–9. DOI: 10.1038/nbt1257. PMID: 17086173.Zwirner K., Thiel C., Thiel K., Morgalla MH., Königsrainer A., Schenk M. 2010. Extracellular brain ammonia levels in association with arterial ammonia, intracranial pressure and the use of albumin dialysis devices in pigs with acute liver failure. Metab Brain Dis. 25:407–12. DOI: 10.1007/s11011-010-9222-x. PMID: 21086032.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Temporal Morphologic Changes in the Mouse Liver after Common Bile Duct Ligation
- Hepatoprotective effect of sodium hydrosulfide on hepatic encephalopathy in rats
- Immunohistochemical Expression of Bcl-2 and Bax after Bile Duct Ligation in the Rat
- Transmission electron microscopic findings of the intra-hepatic biliary tract after bile duct ligation in rats
- Change of Hepatic Volume after Selective Bile Duct Ligation: An Experimental Study in the Rabbit