Cancer Res Treat.
2020 Apr;52(2):469-480. 10.4143/crt.2019.423.
An Integrated Nomogram Combining Clinical Factors andMicrotubule-Associated Protein 1 Light Chain 3B Expression to PredictPostoperative Prognosis in Patients with Intrahepatic Cholangiocarcinoma
- Affiliations
-
- 1Department of Hepatic Surgery and Liver Transplantation Center, Third Affiliated Hospital of Sun Yat-sen University, Guangzhou
- 2Guangdong Key Laboratory of Liver Disease Research, Third Affiliated Hospital of Sun Yat-sen University, Guangzhou
- 3Department of Hepatobiliary Surgery, Affiliated Tumor Hospital of Guangxi Medical University, Nanning, China
- KMID: 2500332
- DOI: http://doi.org/10.4143/crt.2019.423
Abstract
- Purpose
Microtubule-associated protein 1 light chain 3B (LC3B) serves as a key component of autophagy, which is associated with the progression of carcinoma. Yet, it is still unclear whether LC3B is also an independent risk factor for intrahepatic cholangiocarcinoma (ICC). We aim to explore the predictive value of LC3B on prognosis of ICC, and to establish a novel and available nomogram to predict relapse-free survival (RFS) and overall survival (OS) for these patients after curative-intent hepatectomy.
Materials and Methods
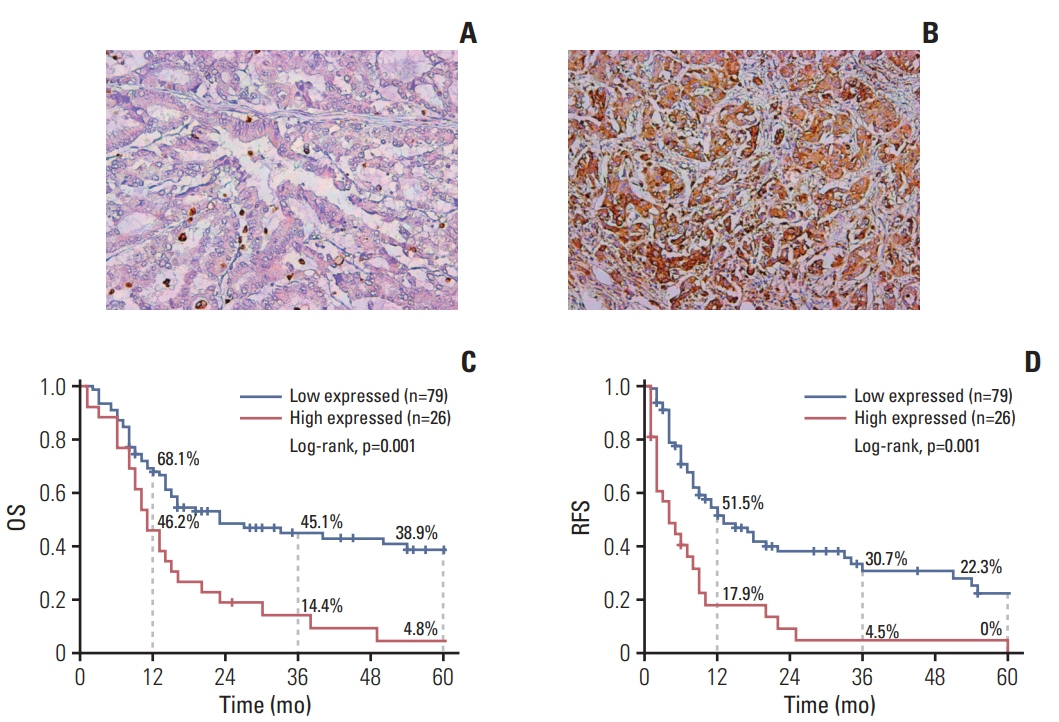
From August 2004 to March 2017, 105 ICC patients were eligibly enrolled in the Third Affiliated Hospital of Sun Yat-sen University. Preoperative clinical information of enrolled patients was collected. Expression LC3B in the ICC specimen was detected by immunohistochemistry.
Results
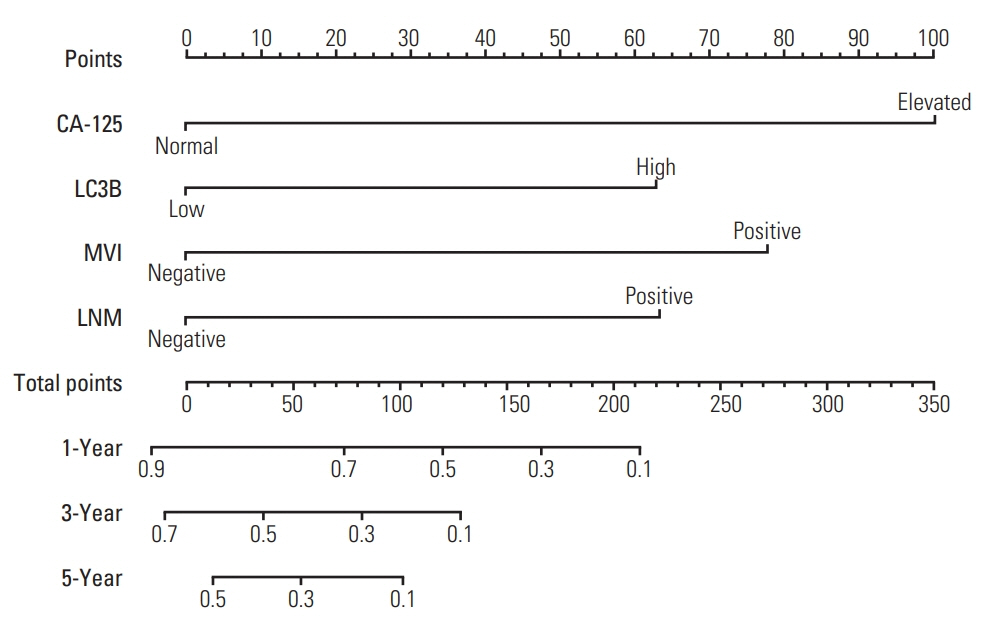
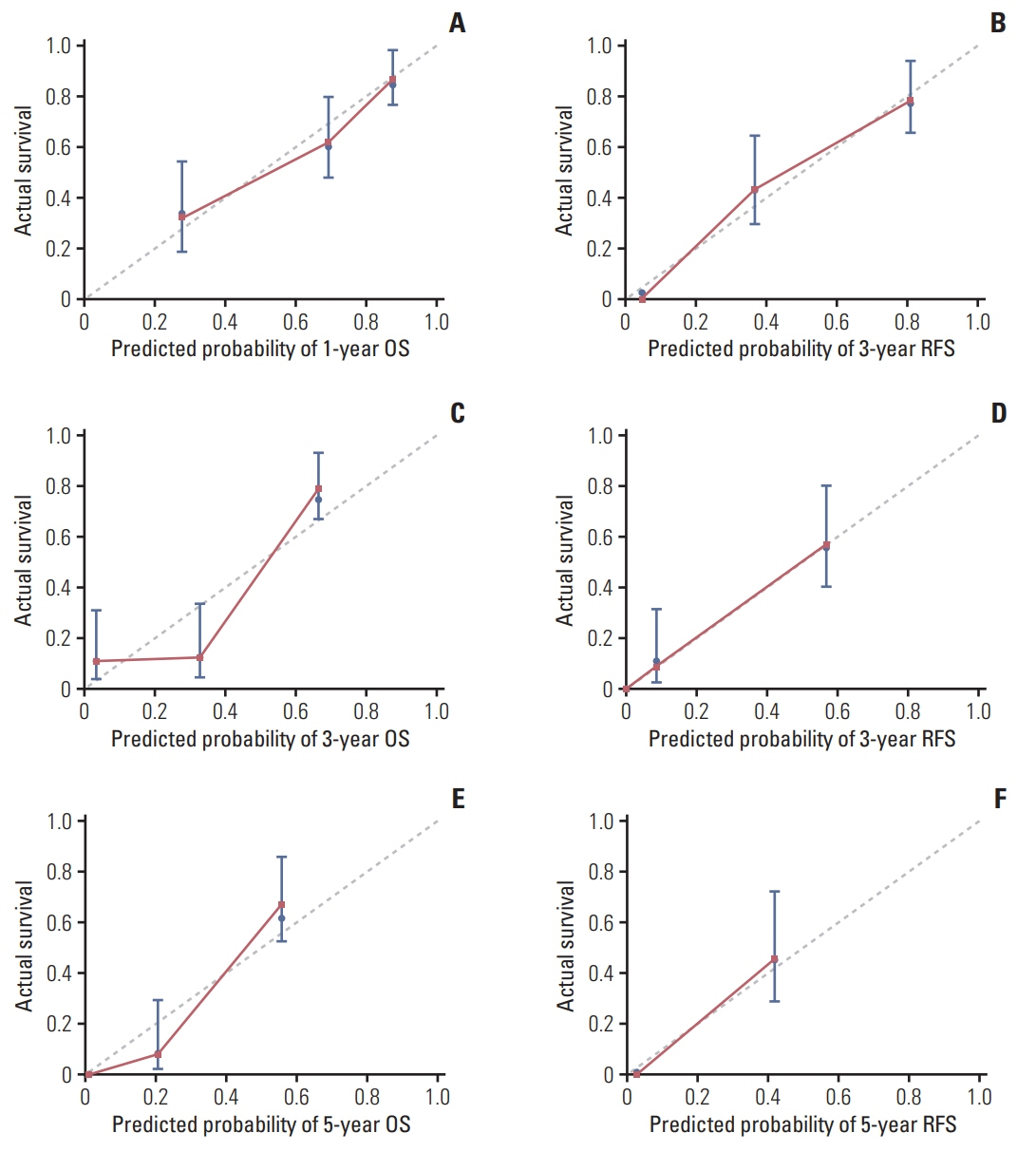
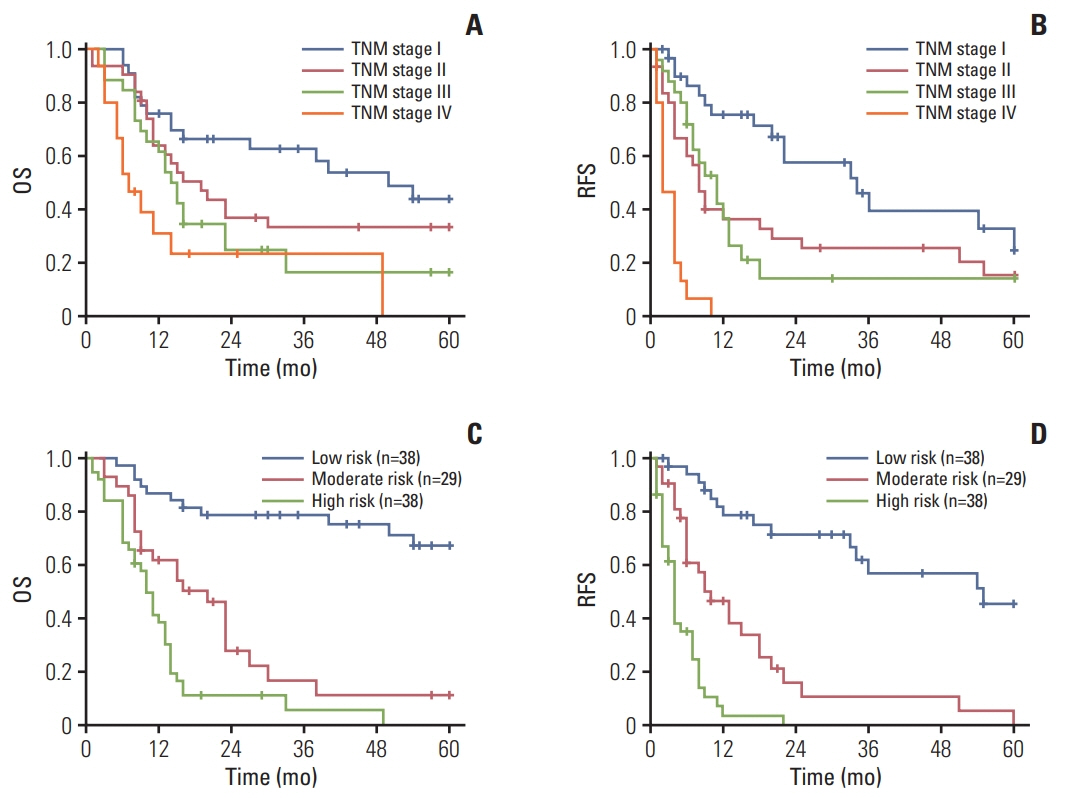
The 5-year RFS and OS in this cohort were 15.7% and 29.6%, respectively. On multivariate Cox regression analysis, independent risk factors for 5-year OS were cancer antigen 125, microvascular invasion, LC3B expression and lymph node metastasis. Except for the above 4 factors, neutrophil/lymphocyte ratio and tumor differentiation were independent factors for 5-year RFS. The area under the curve of nomograms for OS and RFS were 0.820 and 0.747, respectively.
Conclusion
The nomograms based on LC3B can be considered as effective models to predict postoperative survival for ICC patients.
Keyword
Figure
Reference
-
References
1. The general rules for the clinical and pathological study of primary liver cancer. Liver Cancer Study Group of Japan. Jpn J Surg. 1989; 19:98–129.2. Spolverato G, Kim Y, Alexandrescu S, Marques HP, Lamelas J, Aldrighetti L, et al. Management and outcomes of patients with recurrent intrahepatic cholangiocarcinoma following previous curative-intent surgical resection. Ann Surg Oncol. 2016; 23:235–43.
Article3. Zhang H, Yang T, Wu M, Shen F. Intrahepatic cholangiocarcinoma: epidemiology, risk factors, diagnosis and surgical management. Cancer Lett. 2016; 379:198–205.
Article4. Tan JC, Coburn NG, Baxter NN, Kiss A, Law CH. Surgical management of intrahepatic cholangiocarcinoma: a population-based study. Ann Surg Oncol. 2008; 15:600–8.5. Sonbare DJ. Influence of surgical margins on outcome in patients with intrahepatic cholangiocarcinoma: a multicenter study by the AFC-IHCC-2009 Study Group. Ann Surg. 2014; 259:e36.6. Endo I, Gonen M, Yopp AC, Dalal KM, Zhou Q, Klimstra D, et al. Intrahepatic cholangiocarcinoma: rising frequency, improved survival, and determinants of outcome after resection. Ann Surg. 2008; 248:84–96.7. de Jong MC, Nathan H, Sotiropoulos GC, Paul A, Alexandrescu S, Marques H, et al. Intrahepatic cholangiocarcinoma: an international multi-institutional analysis of prognostic factors and lymph node assessment. J Clin Oncol. 2011; 29:3140–5.
Article8. Witjes CD, Karim-Kos HE, Visser O, de Vries E, JN IJ, de Man RA, et al. Intrahepatic cholangiocarcinoma in a low endemic area: rising incidence and improved survival. HPB (Oxford). 2012; 14:777–81.
Article9. Levy JM, Towers CG, Thorburn A. Targeting autophagy in cancer. Nat Rev Cancer. 2017; 17:528–42.
Article10. Lai K, Killingsworth MC, Lee CS. The significance of autophagy in colorectal cancer pathogenesis and implications for therapy. J Clin Pathol. 2014; 67:854–8.
Article11. Nah J, Yuan J, Jung YK. Autophagy in neurodegenerative diseases: from mechanism to therapeutic approach. Mol Cells. 2015; 38:381–9.
Article12. Kondo Y, Kanzawa T, Sawaya R, Kondo S. The role of autophagy in cancer development and response to therapy. Nat Rev Cancer. 2005; 5:726–34.
Article13. Lum JJ, Bauer DE, Kong M, Harris MH, Li C, Lindsten T, et al. Growth factor regulation of autophagy and cell survival in the absence of apoptosis. Cell. 2005; 120:237–48.
Article14. Koukourakis MI, Kalamida D, Giatromanolaki A, Zois CE, Sivridis E, Pouliliou S, et al. Autophagosome proteins LC3A, LC3B and LC3C have distinct subcellular distribution kinetics and expression in cancer cell lines. PLoS One. 2015; 10:e0137675.
Article15. Chen S, Jiang YZ, Huang L, Zhou RJ, Yu KD, Liu Y, et al. The residual tumor autophagy marker LC3B serves as a prognostic marker in local advanced breast cancer after neoadjuvant chemotherapy. Clin Cancer Res. 2013; 19:6853–62.
Article16. Wang JY, Wu T, Ma W, Li S, Jing WJ, Ma J, et al. Expression and clinical significance of autophagic protein LC3B and EMT markers in gastric cancer. Cancer Manag Res. 2018; 10:1479–86.
Article17. Cufi S, Vazquez-Martin A, Oliveras-Ferraros C, MartinCastillo B, Vellon L, Menendez JA. Autophagy positively regulates the CD44(+) CD24(-/low) breast cancer stem-like phenotype. Cell Cycle. 2011; 10:3871–85.18. Mikhaylova O, Stratton Y, Hall D, Kellner E, Ehmer B, Drew AF, et al. VHL-regulated MiR-204 suppresses tumor growth through inhibition of LC3B-mediated autophagy in renal clear cell carcinoma. Cancer Cell. 2012; 21:532–46.
Article19. Chen DP, Ning WR, Li XF, Wei Y, Lao XM, Wang JC, et al. Peritumoral monocytes induce cancer cell autophagy to facilitate the progression of human hepatocellular carcinoma. Autophagy. 2018; 14:1335–46.
Article20. Wang Y, Li J, Xia Y, Gong R, Wang K, Yan Z, et al. Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. J Clin Oncol. 2013; 31:1188–95.
Article21. Hyder O, Marques H, Pulitano C, Marsh JW, Alexandrescu S, Bauer TW, et al. A nomogram to predict long-term survival after resection for intrahepatic cholangiocarcinoma: an Eastern and Western experience. JAMA Surg. 2014; 149:432–8.22. Lefort S, Joffre C, Kieffer Y, Givel AM, Bourachot B, Zago G, et al. Inhibition of autophagy as a new means of improving chemotherapy efficiency in high-LC3B triple-negative breast cancers. Autophagy. 2014; 10:2122–42.
Article23. Remmele W, Hildebrand U, Hienz HA, Klein PJ, Vierbuchen M, Behnken LJ, et al. Comparative histological, histochemical, immunohistochemical and biochemical studies on oestrogen receptors, lectin receptors, and Barr bodies in human breast cancer. Virchows Arch A Pathol Anat Histopathol. 1986; 409:127–47.
Article24. Ogier-Denis E, Codogno P. Autophagy: a barrier or an adaptive response to cancer. Biochim Biophys Acta. 2003; 1603:113–28.
Article25. Lin CI, Whang EE, Donner DB, Du J, Lorch J, He F, et al. Autophagy induction with RAD001 enhances chemosensitivity and radiosensitivity through Met inhibition in papillary thyroid cancer. Mol Cancer Res. 2010; 8:1217–26.
Article26. Zhao R, Bei X, Yang B, Wang X, Jiang C, Shi F, et al. Endothelial cells promote metastasis of prostate cancer by enhancing autophagy. J Exp Clin Cancer Res. 2018; 37:221.
Article27. Hou YJ, Dong LW, Tan YX, Yang GZ, Pan YF, Li Z, et al. Inhibition of active autophagy induces apoptosis and increases chemosensitivity in cholangiocarcinoma. Lab Invest. 2011; 91:1146–57.
Article28. Nitta T, Sato Y, Ren XS, Harada K, Sasaki M, Hirano S, et al. Autophagy may promote carcinoma cell invasion and correlate with poor prognosis in cholangiocarcinoma. Int J Clin Exp Pathol. 2014; 7:4913–21.29. Mizushima N. Methods for monitoring autophagy. Int J Biochem Cell Biol. 2004; 36:2491–502.
Article30. Sato K, Tsuchihara K, Fujii S, Sugiyama M, Goya T, Atomi Y, et al. Autophagy is activated in colorectal cancer cells and contributes to the tolerance to nutrient deprivation. Cancer Res. 2007; 67:9677–84.
Article31. Liu JL, Chen FF, Lung J, Lo CH, Lee FH, Lu YC, et al. Prognostic significance of p62/SQSTM1 subcellular localization and LC3B in oral squamous cell carcinoma. Br J Cancer. 2014; 111:944–54.
Article32. El-Mashed S, O'Donovan TR, Kay EW, Abdallah AR, Cathcart MC, O'Sullivan J, et al. LC3B globular structures correlate with survival in esophageal adenocarcinoma. BMC Cancer. 2015; 15:582.
Article33. Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010; 17:1471–4.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Review: Analysis of Survival Rate and Prognostic Factors of Intrahepatic Cholangiocarcinoma: 318 Cases in Single Institute
- Impact of Nrf2 overexpression on cholangiocarcinoma treatment and clinical prognosis
- Image Findings of Sarcomatous Intrahepatic Cholangiocarcinoma Focused on Gd-EOB-DTPA Enhanced MRI: A Case Report
- Surgical Treatment for Intrahepatic Cholangiocarcinoma
- Expression of p53, Rb, bcl-2 Proteins and Ki-67 Labeling Index in Intrahepatic Cholangiocarcinoma