Dement Neurocogn Disord.
2020 Mar;19(1):1-18. 10.12779/dnd.2020.19.1.1.
Alzheimer's Disease Diagnosis Using Misfolding Proteins in Blood
- Affiliations
-
- 1Integrated Science Engineering Division, Yonsei University, Incheon, Korea. y.kim@yonsei.ac.kr
- 2Department of Pharmacy, Yonsei University, Incheon, Korea.
- 3Yonsei Institute of Pharmaceutical Sciences, Yonsei University, Incheon, Korea.
- 4Underwood Division, Yonsei University, Incheon, Korea.
- 5Yonsei Frontier Lab, Yonsei University, Incheon, Korea.
- 6Pharmaceutical Sciences Division, School of Pharmacy and Carbone Cancer Center, School of Medicine & Public Health, University of Wisconsin, Madison, WI, USA.
- KMID: 2471931
- DOI: http://doi.org/10.12779/dnd.2020.19.1.1
Abstract
- Alzheimer's disease (AD) is pathologically characterized by a long progressive phase of neuronal changes, including accumulation of extracellular amyloid-β (Aβ) and intracellular neurofibrillary tangles, before the onset of observable symptoms. Many efforts have been made to develop a blood-based diagnostic method for AD by incorporating Aβ and tau as plasma biomarkers. As blood tests have the advantages of being highly accessible and low cost, clinical implementation of AD blood tests would provide preventative screening to presymptomatic individuals, facilitating early identification of AD patients and, thus, treatment development in clinical research. However, the low concentration of AD biomarkers in the plasma has posed difficulties for accurate detection, hindering the development of a reliable blood test. In this review, we introduce three AD blood test technologies emerging in South Korea, which have distinctive methods of heightening detection sensitivity of specific plasma biomarkers. We discuss in detail the multimer detection system, the self-standard analysis of Aβ biomarkers quantified by interdigitated microelectrodes, and a biomarker ratio analysis comprising Aβ and tau.
Keyword
MeSH Terms
Figure
Reference
-
1. Hardy JA, Higgins GA. Alzheimer's disease: the amyloid cascade hypothesis. Science. 1992; 256:184–185. PMID: 1566067.
Article2. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984; 34:939–944. PMID: 6610841.
Article3. McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr, Kawas CH, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011; 7:263–269. PMID: 21514250.
Article4. Ewers M, Walsh C, Trojanowski JQ, Shaw LM, Petersen RC, Jack CR Jr, et al. Prediction of conversion from mild cognitive impairment to Alzheimer's disease dementia based upon biomarkers and neuropsychological test performance. Neurobiol Aging. 2012; 33:1203–1214. PMID: 21159408.
Article5. Jacova C, Kertesz A, Blair M, Fisk JD, Feldman HH. Neuropsychological testing and assessment for dementia. Alzheimers Dement. 2007; 3:299–317. PMID: 19595951.
Article6. Morris JC. Clinical dementia rating: a reliable and valid diagnostic and staging measure for dementia of the Alzheimer type. Int Psychogeriatr. 1997; 9 Suppl 1:173–176. PMID: 9447441.
Article7. O'Bryant SE, Waring SC, Cullum CM, Hall J, Lacritz L, Massman PJ, et al. Staging dementia using Clinical Dementia Rating scale sum of boxes scores: a Texas Alzheimer's research consortium study. Arch Neurol. 2008; 65:1091–1095. PMID: 18695059.8. Lynch CA, Walsh C, Blanco A, Moran M, Coen RF, Walsh JB, et al. The clinical dementia rating sum of box score in mild dementia. Dement Geriatr Cogn Disord. 2006; 21:40–43. PMID: 16254429.
Article9. Tombaugh TN, McIntyre NJ. The mini-mental state examination: a comprehensive review. J Am Geriatr Soc. 1992; 40:922–935. PMID: 1512391.
Article10. Harvey PD. Clinical applications of neuropsychological assessment. Dialogues Clin Neurosci. 2012; 14:91–99. PMID: 22577308.
Article11. Kulas JF, Naugle RI. Indications for neuropsychological assessment. Cleve Clin J Med. 2003; 70:785–786. PMID: 14518573.
Article12. Dubois B, Feldman HH, Jacova C, Dekosky ST, Barberger-Gateau P, Cummings J, et al. Research criteria for the diagnosis of Alzheimer's disease: revising the NINCDS-ADRDA criteria. Lancet Neurol. 2007; 6:734–746. PMID: 17616482.
Article13. Du AT, Schuff N, Amend D, Laakso MP, Hsu YY, Jagust WJ, et al. Magnetic resonance imaging of the entorhinal cortex and hippocampus in mild cognitive impairment and Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2001; 71:441–447. PMID: 11561025.
Article14. Bottino CM, Castro CC, Gomes RL, Buchpiguel CA, Marchetti RL, Neto MR. Volumetric MRI measurements can differentiate Alzheimer's disease, mild cognitive impairment, and normal aging. Int Psychogeriatr. 2002; 14:59–72. PMID: 12094908.
Article15. Shivamurthy VK, Tahari AK, Marcus C, Subramaniam RM. Brain FDG PET and the diagnosis of dementia. AJR Am J Roentgenol. 2015; 204:W76–W85. PMID: 25539279.
Article16. Silverman DH, Small GW, Chang CY, Lu CS, Kung De Aburto MA, Chen W, et al. Positron emission tomography in evaluation of dementia: Regional brain metabolism and long-term outcome. JAMA. 2001; 286:2120–2127. PMID: 11694153.17. Saint-Aubert L, Lemoine L, Chiotis K, Leuzy A, Rodriguez-Vieitez E, Nordberg A. Tau PET imaging: present and future directions. Mol Neurodegener. 2017; 12:19. PMID: 28219440.
Article18. Villemagne VL, Doré V, Burnham SC, Masters CL, Rowe CC. Imaging tau and amyloid-β proteinopathies in Alzheimer disease and other conditions. Nat Rev Neurol. 2018; 14:225–236. PMID: 29449700.
Article19. Anoop A, Singh PK, Jacob RS, Maji SK. CSF biomarkers for Alzheimer's disease diagnosis. Int J Alzheimers Dis. 2010; 2010:606802. PMID: 20721349.
Article20. Tapiola T, Alafuzoff I, Herukka SK, Parkkinen L, Hartikainen P, Soininen H, et al. Cerebrospinal fluid β-amyloid 42 and tau proteins as biomarkers of Alzheimer-type pathologic changes in the brain. Arch Neurol. 2009; 66:382–389. PMID: 19273758.
Article21. Jack CR Jr, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, et al. NIA-AA Research Framework: toward a biological definition of Alzheimer's disease. Alzheimers Dement. 2018; 14:535–562. PMID: 29653606.
Article22. Jack CR Jr, Bennett DA, Blennow K, Carrillo MC, Feldman HH, Frisoni GB, et al. A/T/N: an unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology. 2016; 87:539–547. PMID: 27371494.
Article23. Shibata M, Yamada S, Kumar SR, Calero M, Bading J, Frangione B, et al. Clearance of Alzheimer's amyloid-β1–40 peptide from brain by LDL receptor-related protein-1 at the blood-brain barrier. J Clin Invest. 2000; 106:1489–1499. PMID: 11120756.24. van de Haar HJ, Burgmans S, Jansen JF, van Osch MJ, van Buchem MA, Muller M, et al. Blood-brain barrier leakage in patients with early Alzheimer disease. Radiology. 2016; 281:527–535. PMID: 27243267.
Article25. Issaq HJ, Xiao Z, Veenstra TD. Serum and plasma proteomics. Chem Rev. 2007; 107:3601–3620. PMID: 17636887.
Article26. O'Bryant SE, Edwards M, Johnson L, Hall J, Villarreal AE, Britton GB, et al. A blood screening test for Alzheimer's disease. Alzheimers Dement (Amst). 2016; 3:83–90. PMID: 27453929.27. Mehta PD, Pirttilä T, Mehta SP, Sersen EA, Aisen PS, Wisniewski HM. Plasma and cerebrospinal fluid levels of amyloid β proteins 1–40 and 1–42 in Alzheimer disease. Arch Neurol. 2000; 57:100–105. PMID: 10634455.
Article28. Zecca C, Tortelli R, Panza F, Arcuti S, Piccininni M, Capozzo R, et al. Plasma β-amyloid1–42 reference values in cognitively normal subjects. J Neurol Sci. 2018; 391:120–126. PMID: 30103961.29. Tatebe H, Kasai T, Ohmichi T, Kishi Y, Kakeya T, Waragai M, et al. Quantification of plasma phosphorylated tau to use as a biomarker for brain Alzheimer pathology: pilot case-control studies including patients with Alzheimer's disease and down syndrome. Mol Neurodegener. 2017; 12:63. PMID: 28866979.
Article30. Zetterberg H, Wilson D, Andreasson U, Minthon L, Blennow K, Randall J, et al. Plasma tau levels in Alzheimer's disease. Alzheimers Res Ther. 2013; 5:9. PMID: 23551972.
Article31. Lee JC, Kim SJ, Hong S, Kim Y. Diagnosis of Alzheimer's disease utilizing amyloid and tau as fluid biomarkers. Exp Mol Med. 2019; 51:53.
Article32. Thambisetty M, Lovestone S. Blood-based biomarkers of Alzheimer's disease: challenging but feasible. Biomarkers Med. 2010; 4:65–79.
Article33. Kuo YM, Kokjohn TA, Kalback W, Luehrs D, Galasko DR, Chevallier N, et al. Amyloid-β peptides interact with plasma proteins and erythrocytes: implications for their quantitation in plasma. Biochem Biophys Res Commun. 2000; 268:750–756. PMID: 10679277.
Article34. Pencina MJ, D'Agostino RB Sr, D'Agostino RB Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008; 27:157–172. PMID: 17569110.
Article35. Lalkhen AG, McCluskey A. Clinical tests: sensitivity and specificity. Contin Educ Anaesth Crit Care Pain. 2008; 8:221–223.
Article36. The Ronald and Nancy Reagan Research Institute of the Alzheimer's Association and the National Institute on Aging Working Group. Consensus report of the Working Group on: “Molecular and Biochemical Markers of Alzheimer's Disease”. Neurobiol Aging. 1998; 19:109–116. PMID: 9558143.37. Pesini P, Pérez-Grijalba V, Monleón I, Boada M, Tárraga L, Martínez-Lage P, et al. Reliable measurements of the β-amyloid Pool in blood could help in the early diagnosis of AD. Int J Alzheimers Dis. 2012; 2012:604141. PMID: 22957297.38. Pérez-Grijalba V, Pesini P, Monleón I, Boada M, Tárraga L, Ruiz-Laza A, et al. Several direct and calculated biomarkers from the amyloid-β pool in blood are associated with an increased likelihood of suffering from mild cognitive impairment. J Alzheimers Dis. 2013; 36:211–219. PMID: 23635404.
Article39. Bateman RJ, Munsell LY, Morris JC, Swarm R, Yarasheski KE, Holtzman DM. Human amyloid-beta synthesis and clearance rates as measured in cerebrospinal fluid in vivo . Nat Med. 2006; 12:856–861. PMID: 16799555.40. Ovod V, Ramsey KN, Mawuenyega KG, Bollinger JG, Hicks T, Schneider T, et al. Amyloid β concentrations and stable isotope labeling kinetics of human plasma specific to central nervous system amyloidosis. Alzheimers Dement. 2017; 13:841–849. PMID: 28734653.
Article41. Yang CC, Yang SY, Chieh JJ, Horng HE, Hong CY, Yang HC, et al. Biofunctionalized magnetic nanoparticles for specifically detecting biomarkers of Alzheimer's disease in vitro. ACS Chem Neurosci. 2011; 2:500–505. PMID: 22860173.
Article42. Chieh JJ, Yang SY, Jian Z, Wang W, Horng HE, Yang HC, et al. Hyper-high-sensitivity wash-free magnetoreduction assay on biomolecules using high-Tc superconducting quantum interference devices. J Appl Phys. 2008; 103:014703.43. Lue LF, Guerra A, Walker DG. Amyloid beta and tau as Alzheimer's disease blood biomarkers: promise from new technologies. Neurol Ther. 2017; 6:25–36.
Article44. Chiu MJ, Yang SY, Horng HE, Yang CC, Chen TF, Chieh JJ, et al. Combined plasma biomarkers for diagnosing mild cognition impairment and Alzheimer's disease. ACS Chem Neurosci. 2013; 4:1530–1536. PMID: 24090201.
Article45. Hye A, Lynham S, Thambisetty M, Causevic M, Campbell J, Byers HL, et al. Proteome-based plasma biomarkers for Alzheimer's disease. Brain. 2006; 129:3042–3050. PMID: 17071923.
Article46. Thambisetty M, Tripaldi R, Riddoch-Contreras J, Hye A, An Y, Campbell J, et al. Proteome-based plasma markers of brain amyloid-β deposition in non-demented older individuals. J Alzheimers Dis. 2010; 22:1099–1109. PMID: 20930274.
Article47. Thambisetty M, Simmons A, Velayudhan L, Hye A, Campbell J, Zhang Y, et al. Association of plasma clusterin concentration with severity, pathology, and progression in Alzheimer disease. Arch Gen Psychiatry. 2010; 67:739–748. PMID: 20603455.
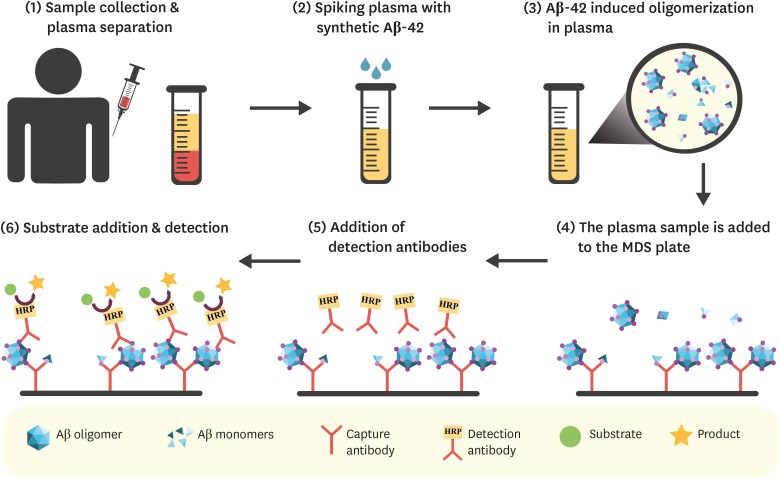
Article48. An SS, Lee BS, Yu JS, Lim K, Kim GJ, Lee R, et al. Dynamic changes of oligomeric amyloid β levels in plasma induced by spiked synthetic Aβ42 . Alzheimers Res Ther. 2017; 9:86. PMID: 29041968.
Article49. An SS, Lim KT, Oh HJ, Lee BS, Zukic E, Ju YR, et al. Differentiating blood samples from scrapie infected and non-infected hamsters by detecting disease-associated prion proteins using multimer detection system. Biochem Biophys Res Commun. 2010; 392:505–509. PMID: 20085753.
Article50. Wang MJ, Yi S, Han JY, Park SY, Jang JW, Chun IK, et al. Oligomeric forms of amyloid-β protein in plasma as a potential blood-based biomarker for Alzheimer's disease. Alzheimers Res Ther. 2017; 9:98. PMID: 29246249.
Article51. Youn YC, Kang S, Suh J, Park YH, Kang MJ, Pyun JM, et al. Blood amyloid-β oligomerization associated with neurodegeneration of Alzheimer's disease. Alzheimers Res Ther. 2019; 11:40. PMID: 31077246.
Article52. De Felice FG, Wu D, Lambert MP, Fernandez SJ, Velasco PT, Lacor PN, et al. Alzheimer's disease-type neuronal tau hyperphosphorylation induced by Aβ oligomers. Neurobiol Aging. 2008; 29:1334–1347. PMID: 17403556.
Article53. Sakono M, Zako T. Amyloid oligomers: formation and toxicity of Aβ oligomers. FEBS J. 2010; 277:1348–1358. PMID: 20148964.
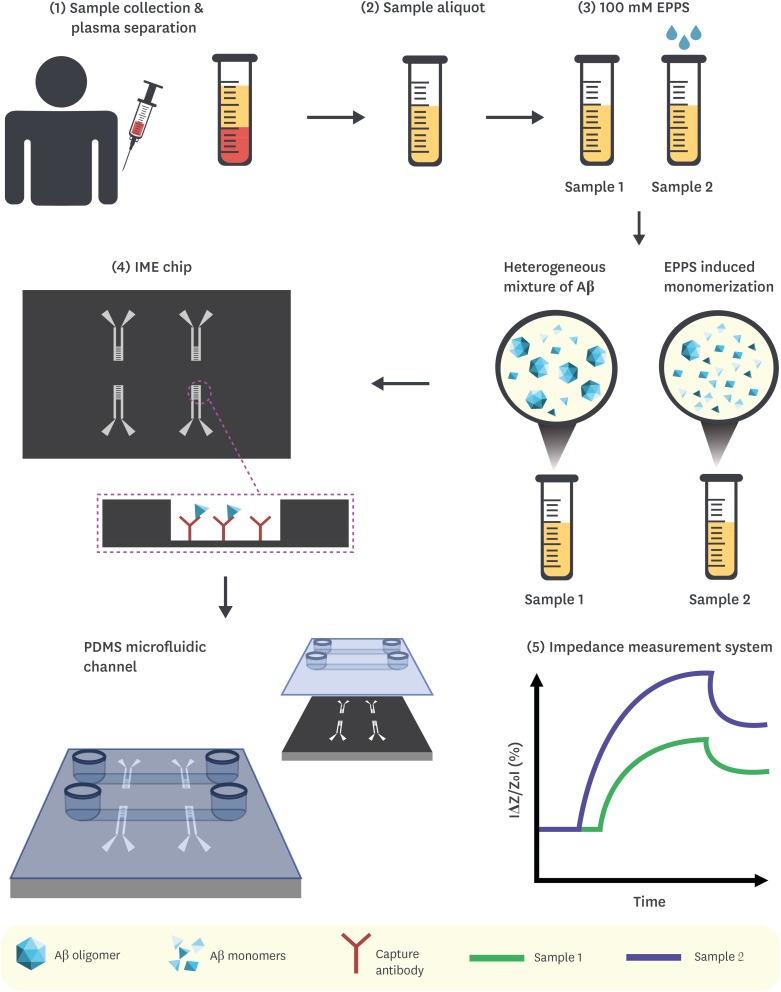
Article54. SS AnKT LimHJ Oh. Peoplebio, Inc.Differential detection of multimeric and monomeric forms of multimer-forming polypeptides. United States patent US 8,026,070 B2. 2011. 9. 27.55. Kim Y, Yoo YK, Kim HY, Roh JH, Kim J, Baek S, et al. Comparative analyses of plasma amyloid-β levels in heterogeneous and monomerized states by interdigitated microelectrode sensor system. Sci Adv. 2019; 5:eaav1388. PMID: 31001580.
Article56. Kim HY, Kim HV, Jo S, Lee CJ, Choi SY, Kim DJ, et al. EPPS rescues hippocampus-dependent cognitive deficits in APP/PS1 mice by disaggregation of amyloid-β oligomers and plaques. Nat Commun. 2015; 6:8997. PMID: 26646366.
Article57. Yoo YK, Kim J, Kim G, Kim YS, Kim HY, Lee S, et al. A highly sensitive plasma-based amyloid-β detection system through medium-changing and noise cancellation system for early diagnosis of the Alzheimer's disease. Sci Rep. 2017; 7:8882. PMID: 28827785.
Article58. Yoo YK, Yoon DS, Kim G, Kim J, Han SI, Lee J, et al. An enhanced platform to analyse low-affinity amyloid β protein by integration of electrical detection and preconcentrator. Sci Rep. 2017; 7:14303. PMID: 29084978.
Article59. Huang Y, Potter R, Sigurdson W, Kasten T, Connors R, Morris JC, et al. β-amyloid dynamics in human plasma. Arch Neurol. 2012; 69:1591–1597. PMID: 23229043.
Article60. Roher AE, Esh CL, Kokjohn TA, Castaño EM, Van Vickle GD, Kalback WM, et al. Amyloid beta peptides in human plasma and tissues and their significance for Alzheimer's disease. Alzheimers Dement. 2009; 5:18–29. PMID: 19118806.
Article61. Villemagne VL, Ong K, Mulligan RS, Holl G, Pejoska S, Jones G, et al. Amyloid imaging with (8F-florbetaben in Alzheimer disease and other dementias. J Nucl Med. 2011; 52:1210–1217. PMID: 21764791.62. Oh SJ, Kim MH, Han SJ, Kang KJ, Ko IO, Kim Y, et al. Preliminary PET study of 18F-FC119S in normal and Alzheimer's disease models. Mol Pharm. 2017; 14:3114–3120. PMID: 28737945.63. Park JC, Han SH, Yi D, Byun MS, Lee JH, Jang S, et al. Plasma tau/amyloid-β1-42 ratio predicts brain tau deposition and neurodegeneration in Alzheimer's disease. Brain. 2019; 142:771–786. PMID: 30668647.
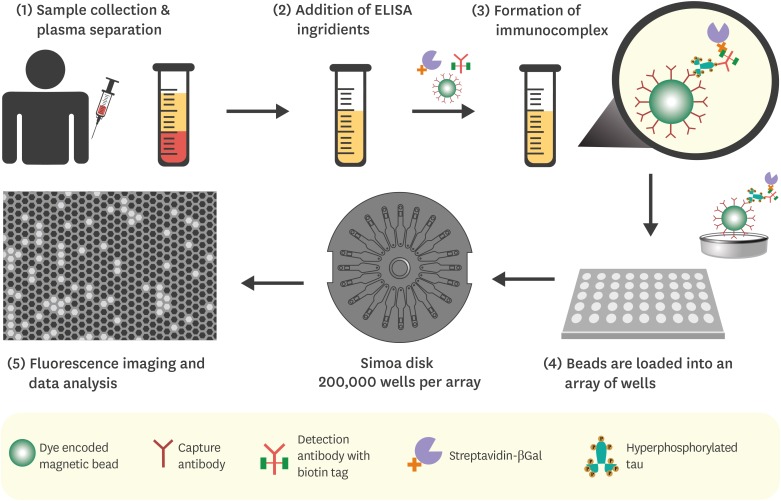
Article64. Wilson DH, Rissin DM, Kan CW, Fournier DR, Piech T, Campbell TG, et al. The Simoa HD-1 analyzer: a novel fully automated digital immunoassay analyzer with single-molecule sensitivity and multiplexing. J Lab Autom. 2016; 21:533–547. PMID: 26077162.65. Rissin DM, Kan CW, Campbell TG, Howes SC, Fournier DR, Song L, et al. Single-molecule enzyme-linked immunosorbent assay detects serum proteins at subfemtomolar concentrations. Nat Biotechnol. 2010; 28:595–599. PMID: 20495550.
Article66. Fischer SK, Joyce A, Spengler M, Yang TY, Zhuang Y, Fjording MS, et al. Emerging technologies to increase ligand binding assay sensitivity. AAPS J. 2015; 17:93–101. PMID: 25331105.
Article67. Fulton RJ, McDade RL, Smith PL, Kienker LJ, Kettman JR Jr. Advanced multiplexed analysis with the FlowMetrix system. Clin Chem. 1997; 43:1749–1756. PMID: 9299971.68. Reslova N, Michna V, Kasny M, Mikel P, Kralik P. xMAP technology: applications in detection of pathogens. Front Microbiol. 2017; 8:55. PMID: 28179899.
Article69. Park JC, Han SH, Cho HJ, Byun MS, Yi D, Choe YM, et al. Chemically treated plasma Aβ is a potential blood-based biomarker for screening cerebral amyloid deposition. Alzheimers Res Ther. 2017; 9:20. PMID: 28330509.
Article70. Baker SL, Maass A, Jagust WJ. Considerations and code for partial volume correcting [18F]-AV-1451 tau PET data. Data Brief. 2017; 15:648–657. PMID: 29124088.71. Braak H, Braak E. Staging of Alzheimer's disease-related neurofibrillary changes. Neurobiol Aging. 1995; 16:271–278. PMID: 7566337.
Article72. Dage JL, Wennberg AM, Airey DC, Hagen CE, Knopman DS, Machulda MM, et al. Levels of tau protein in plasma are associated with neurodegeneration and cognitive function in a population-based elderly cohort. Alzheimers Dement. 2016; 12:1226–1234. PMID: 27436677.
Article73. Mattsson N, Zetterberg H, Janelidze S, Insel PS, Andreasson U, Stomrud E, et al. Plasma tau in Alzheimer disease. Neurology. 2016; 87:1827–1835. PMID: 27694257.
Article74. Fagan AM, Roe CM, Xiong C, Mintun MA, Morris JC, Holtzman DM. Cerebrospinal fluid tau/β-amyloid42 ratio as a prediction of cognitive decline in nondemented older adults. Arch Neurol. 2007; 64:343–349. PMID: 17210801.75. Gómez-Tortosa E, Gonzalo I, Fanjul S, Sainz MJ, Cantarero S, Cemillán C, et al. Cerebrospinal fluid markers in dementia with Lewy bodies compared with Alzheimer disease. Arch Neurol. 2003; 60:1218–1222. PMID: 12975286.
Article76. Ritchie C, Smailagic N, Noel-Storr AH, Ukoumunne O, Ladds EC, Martin S. CSF tau and the CSF tau/Aβ ratio for the diagnosis of Alzheimer's disease dementia and other dementias in people with mild cognitive impairment (MCI). Cochrane Database Syst Rev. 2017; 3:CD010803. PMID: 28328043.77. Olsson A, Vanderstichele H, Andreasen N, De Meyer G, Wallin A, Holmberg B, et al. Simultaneous measurement of β -amyloid1– 42, total tau, and phosphorylated tau (Thr181) in cerebrospinal fluid by the xMAP technology. Clin Chem. 2005; 51:336–345. PMID: 15563479.78. Bateman RJ, Xiong C, Benzinger TL, Fagan AM, Goate A, Fox NC, et al. Clinical and biomarker changes in dominantly inherited Alzheimer's disease. N Engl J Med. 2012; 367:795–804. PMID: 22784036.
Article79. Zissimopoulos J, Crimmins E, St Clair P. The value of delaying Alzheimer's disease onset. Forum Health Econ Policy. 2014; 18:25–39. PMID: 27134606.
Article80. Zemek F, Drtinova L, Nepovimova E, Sepsova V, Korabecny J, Klimes J, et al. Outcomes of Alzheimer's disease therapy with acetylcholinesterase inhibitors and memantine. Expert Opin Drug Saf. 2014; 13:759–774. PMID: 24845946.81. Jo K, Jhoo JH, Mun YJ, Kim YM, Kim SK, Kim S, et al. The effect of cognitive intervention on cognitive improvement in patients with dementia. Dement Neurocogn Disord. 2018; 17:23–31. PMID: 30906388.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Misfolded Proteins in Neurodegenerative Dementias: Molecular Mechanisms
- Pathobiolgy and Management of Alzheimer’s Disease
- Memorials of Alois Alzheimer (June 14, 1864~December 19, 1915) and Historical Background of Alzheimer's Disease
- Significance of Non-Alzheimer Dementia
- Diagnosis and Treatment of Alzheimer’s Disease: Current Update