J Gastric Cancer.
2020 Mar;20(1):60-71. 10.5230/jgc.2020.20.e7.
Establishment of a [¹â¸F]-FDG-PET/MRI Imaging Protocol for Gastric Cancer PDX as a Preclinical Research Tool
- Affiliations
-
- 1Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea. hkyang@snu.ac.kr
- 2Department of Surgery, Seoul National University Hospital, Seoul, Korea.
- 3Department of General, Visceral and Transplant Surgery, University of Mainz, Mainz, Germany.
- 4Department of Pathology, Seoul National University College of Medicine, Seoul, Korea.
- 5Department of Nuclear Medicine, Seoul National University Hospital, Seoul, Korea.
- KMID: 2471926
- DOI: http://doi.org/10.5230/jgc.2020.20.e7
Abstract
- PURPOSE
The utility of 18-fluordesoxyglucose positron emission tomography ([¹â¸F]-FDG-PET) combined with computer tomography or magnetic resonance imaging (MRI) in gastric cancer remains controversial and a rationale for patient selection is desired. This study aims to establish a preclinical patient-derived xenograft (PDX) based [¹â¸F]-FDG-PET/MRI protocol for gastric cancer and compare different PDX models regarding tumor growth and FDG uptake.
MATERIALS AND METHODS
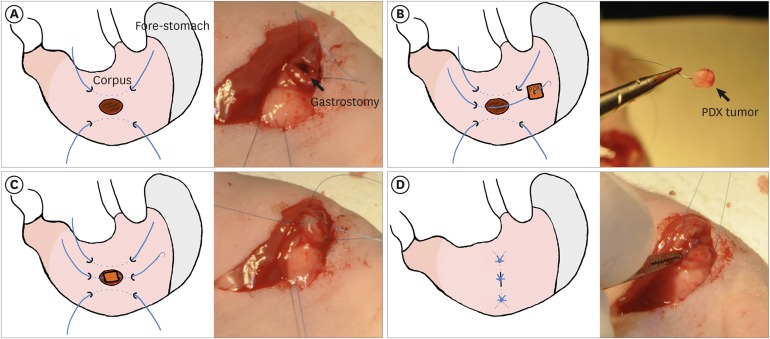
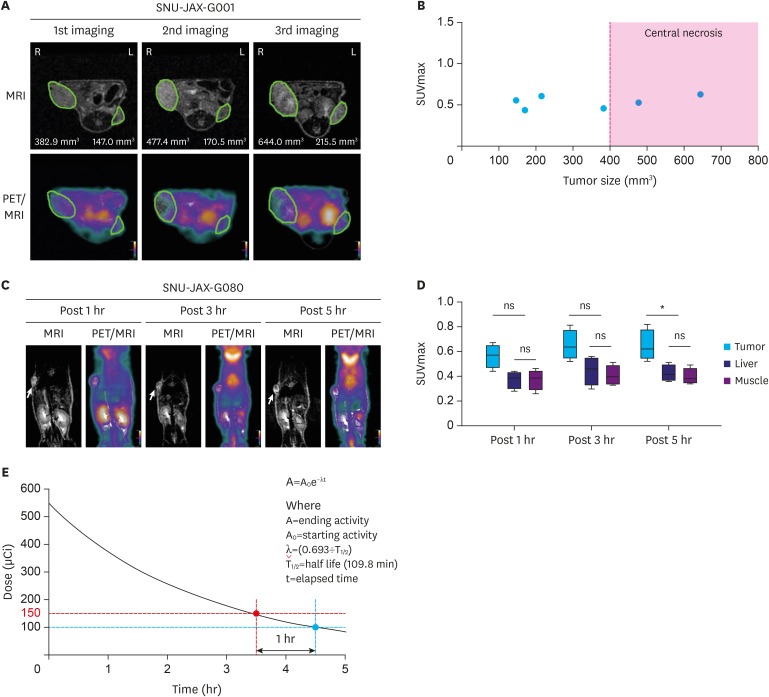
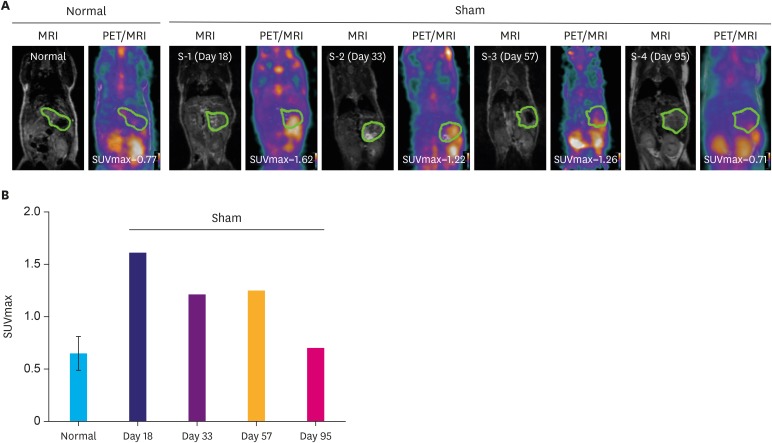
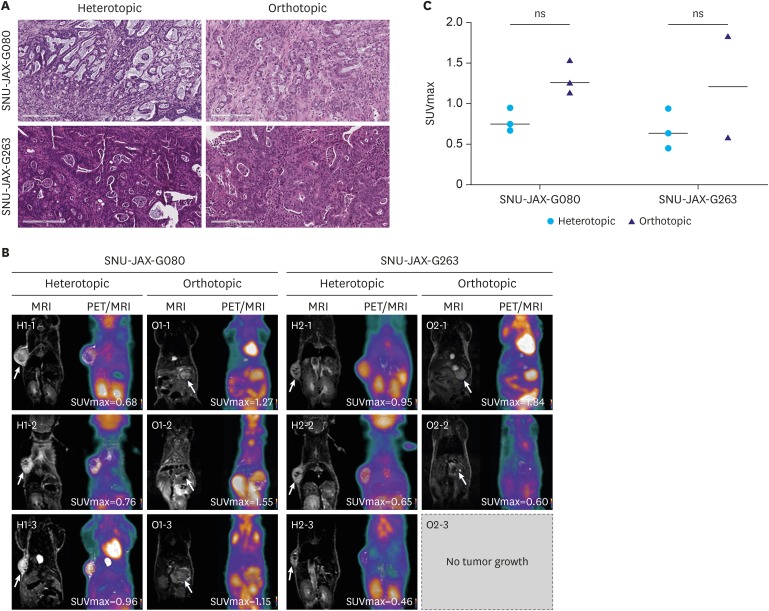
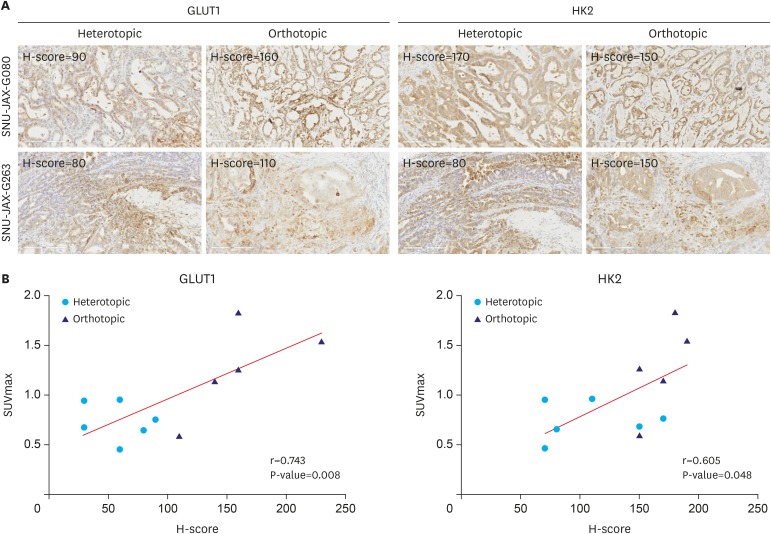
Female BALB/c nu/nu mice were implanted orthotopically and subcutaneously with gastric cancer PDX. [¹â¸F]-FDG-PET/MRI scanning protocol evaluation included different tumor sizes, FDG doses, scanning intervals, and organ-specific uptake. FDG avidity of similar PDX cases were compared between ortho- and heterotopic tumor implantation methods. Microscopic and immunohistochemical investigations were performed to confirm tumor growth and correlate the glycolysis markers glucose transporter 1 (GLUT1) and hexokinase 2 (HK2) with FDG uptake.
RESULTS
Organ-specific uptake analysis showed specific FDG avidity of the tumor tissue. Standard scanning protocol was determined to include 150 μCi FDG injection dose and scanning after one hour. Comparison of heterotopic and orthotopic implanted mice revealed a long growth interval for orthotopic models with a high uptake in similar PDX tissues. The H-score of GLUT1 and HK2 expression in tumor cells correlated with the measured maximal standardized uptake value values (GLUT1: Pearson r=0.743, P=0.009; HK2: Pearson r=0.605, P=0.049).
CONCLUSIONS
This preclinical gastric cancer PDX based [¹â¸F]-FDG-PET/MRI protocol reveals tumor specific FDG uptake and shows correlation to glucose metabolic proteins. Our findings provide a PET/MRI PDX model that can be applicable for translational gastric cancer research.
Keyword
MeSH Terms
Figure
Reference
-
1. Gullo I, Carneiro F, Oliveira C, Almeida GM. Heterogeneity in gastric cancer: from pure morphology to molecular classifications. Pathobiology. 2018; 85:50–63. PMID: 28618420.
Article2. Schlößer HA, Drebber U, Urbanski A, Haase S, Baltin C, Berlth F, et al. Glucose transporters 1, 3, 6, and 10 are expressed in gastric cancer and glucose transporter 3 is associated with UICC stage and survival. Gastric Cancer. 2017; 20:83–91. PMID: 26643879.
Article3. Berlth F, Mönig SP, Schlösser HA, Maus M, Baltin CT, Urbanski A, et al. Validation of 2-mm tissue microarray technology in gastric cancer. Agreement of 2-mm TMAs and full sections for GLUT-1 and HIF-1 alpha. Anticancer Res. 2014; 34:3313–3320. PMID: 24982335.4. Alakus H, Batur M, Schmidt M, Drebber U, Baldus SE, Vallböhmer D, et al. Variable 18F-fluorodeoxyglucose uptake in gastric cancer is associated with different levels of GLUT-1 expression. Nucl Med Commun. 2010; 31:532–538. PMID: 20220543.
Article5. Berlth F, Mönig S, Pinther B, Grimminger P, Maus M, Schlösser H, et al. Both GLUT-1 and GLUT-14 are independent prognostic factors in gastric adenocarcinoma. Ann Surg Oncol. 2015; 22(Suppl 3):S822–S831. PMID: 26183839.
Article6. Chon HJ, Kim C, Cho A, Kim YM, Jang SJ, Kim BO, et al. The clinical implications of FDG-PET/CT differ according to histology in advanced gastric cancer. Gastric Cancer. 2019; 22:113–122. PMID: 29948387.
Article7. Findlay JM, Antonowicz S, Segaran A, El Kafsi J, Zhang A, Bradley KM, et al. Routinely staging gastric cancer with 18F-FDG PET-CT detects additional metastases and predicts early recurrence and death after surgery. Eur Radiol. 2019; 29:2490–2498. PMID: 30643947.8. Borggreve AS, Goense L, Brenkman HJ, Mook S, Meijer GJ, Wessels FJ, et al. Imaging strategies in the management of gastric cancer: current role and future potential of MRI. Br J Radiol. 2019; 92:20181044. PMID: 30789792.
Article9. Brenkman HJ, Gertsen EC, Vegt E, van Hillegersberg R, van Berge Henegouwen MI, Gisbertz SS, et al. Evaluation of PET and laparoscopy in STagIng advanced gastric cancer: a multicenter prospective study (PLASTIC-study). BMC Cancer. 2018; 18:450. PMID: 29678145.
Article10. Lai Y, Wei X, Lin S, Qin L, Cheng L, Li P. Current status and perspectives of patient-derived xenograft models in cancer research. J Hematol Oncol. 2017; 10:106. PMID: 28499452.
Article11. Choi YY, Lee JE, Kim H, Sim MH, Kim KK, Lee G, et al. Establishment and characterisation of patient-derived xenografts as paraclinical models for gastric cancer. Sci Rep. 2016; 6:22172. PMID: 26926953.
Article12. Sulaiman A, Wang L. Bridging the divide: preclinical research discrepancies between triple-negative breast cancer cell lines and patient tumors. Oncotarget. 2017; 8:113269–113281. PMID: 29348905.
Article13. Fueger BJ, Czernin J, Hildebrandt I, Tran C, Halpern BS, Stout D, et al. Impact of animal handling on the results of 18F-FDG PET studies in mice. J Nucl Med. 2006; 47:999–1006. PMID: 16741310.14. Ko GB, Yoon HS, Kim KY, Lee MS, Yang BY, Jeong JM, et al. Simultaneous multiparametric PET/MRI with silicon photomultiplier PET and ultra-high-field MRI for small-animal imaging. J Nucl Med. 2016; 57:1309–1315. PMID: 27081173.
Article15. Jacobson O, Chen X. PET designated flouride-18 production and chemistry. Curr Top Med Chem. 2010; 10:1048–1059. PMID: 20388116.
Article16. Goulding H, Pinder S, Cannon P, Pearson D, Nicholson R, Snead D, et al. A new immunohistochemical antibody for the assessment of estrogen receptor status on routine formalin-fixed tissue samples. Hum Pathol. 1995; 26:291–294. PMID: 7890280.
Article17. Lu P, Yu L, Li Y, Sun Y. A correlation study between maximum standardized uptake values and pathology and clinical staging in nonsmall cell lung cancer. Nucl Med Commun. 2010; 31:646–651. PMID: 20545045.
Article18. Khalaf M, Abdel-Nabi H, Baker J, Shao Y, Lamonica D, Gona J. Relation between nodule size and 18F-FDG-PET SUV for malignant and benign pulmonary nodules. J Hematol Oncol. 2008; 1:13. PMID: 18808716.
Article19. Pleitz JL, Sinha P, Dressler EV, Aouad RK. Correlation of positron emission tomography/computed tomography scan with smoking, tumor size, stage and differentiation in head and neck cancer patients. World J Nucl Med. 2017; 16:51–55. PMID: 28217020.20. Tian J, Chen L, Wei B, Shao M, Ding Y, Yin D, et al. The value of vesicant 18F-fluorodeoxyglucose positron emission tomography (18F-FDG PET) in gastric malignancies. Nucl Med Commun. 2004; 25:825–831. PMID: 15266178.
Article21. Kim SK, Kang KW, Lee JS, Kim HK, Chang HJ, Choi JY, et al. Assessment of lymph node metastases using 18F-FDG PET in patients with advanced gastric cancer. Eur J Nucl Med Mol Imaging. 2006; 33:148–155. PMID: 16228236.22. Kim HW, Won KS, Song BI, Kang YN. Correlation of primary tumor FDG uptake with histopathologic features of advanced gastric cancer. Nucl Med Mol Imaging. 2015; 49:135–142. PMID: 26085859.
Article23. Miao Y, Zhang LF, Guo R, Liang S, Zhang M, Shi S, et al. 18F-FDG PET/CT for monitoring the response of breast cancer to miR-143-based therapeutics by targeting tumor glycolysis. Mol Ther Nucleic Acids. 2016; 5:e357. PMID: 27574783.24. Fledelius J, Winther-Larsen A, Khalil AA, Bylov CM, Hjorthaug K, Bertelsen A, et al. 18F-FDG PET/CT for very early response evaluation predicts CT response in erlotinib-treated non-small cell lung cancer patients: a comparison of assessment methods. J Nucl Med. 2017; 58:1931–1937. PMID: 28490472.25. Valtorta S, Moro M, Prisinzano G, Bertolini G, Tortoreto M, Raccagni I, et al. Metabolic evaluation of non-small cell lung cancer patient-derived xenograft models using 18F-FDG PET: a potential tool for early therapy response. J Nucl Med. 2017; 58:42–47. PMID: 27765858.26. Haldorsen IS, Popa M, Fonnes T, Brekke N, Kopperud R, Visser NC, et al. Multimodal imaging of orthotopic mouse model of endometrial carcinoma. PLoS One. 2015; 10:e0135220. PMID: 26252891.
Article27. Partecke IL, Kaeding A, Sendler M, Albers N, Kühn JP, Speerforck S, et al. In vivo imaging of pancreatic tumours and liver metastases using 7 Tesla MRI in a murine orthotopic pancreatic cancer model and a liver metastases model. BMC Cancer. 2011; 11:40. PMID: 21276229.
Article28. Ramasawmy R, Johnson SP, Roberts TA, Stuckey DJ, David AL, Pedley RB, et al. Monitoring the growth of an orthotopic tumour xenograft model: multi-modal imaging assessment with benchtop MRI (1T), high-field MRI (9.4T), ultrasound and bioluminescence. PLoS One. 2016; 11:e0156162. PMID: 27223614.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- ¹â¸F-FDG PET/MR Refines Evaluation in Newly Diagnosed Metastatic Urethral Adenocarcinoma
- Role of ¹â¸F-FDG PET-CT in Monitoring the Cyclophosphamide Induced Pulmonary Toxicity in Patients with Breast Cancer: 2 Case Reports
- FDG Whole-Body PET/MRI in Oncology: a Systematic Review
- Role of PET Scan in Gastric Cancer as a Diagnostic Tool
- â¶â¸Gallium-Arginine-Glycine-Aspartic Acid and ¹â¸F-Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography in Chondroblastic Osteosarcoma of the Skull