World J Mens Health.
2020 Apr;38(2):198-207. 10.5534/wjmh.180099.
New Insights on the Mechanisms Affecting Fertility in Men with Non-Seminoma Testicular Cancer before Cancer Therapy
- Affiliations
-
- 1American Center for Reproductive Medicine, Cleveland Clinic, Cleveland, OH, USA. agarwaa@ccf.org
- 2Universidade da Beira Interior, Covilhã, Portugal.
- 3Department of Microscopy, Laboratory of Cell Biology, Institute of Biomedical Sciences Abel Salazar and Unit for Multidisciplinary Research in Biomedicine, University of Porto, Porto, Portugal.
- 4Center of Excellence in Genomic Medicine Research, Faculty of Applied Medical Sciences, Jeddah, Saudi Arabia.
- 5Division of Pathology, School of Medical Sciences, Sydney University, Sydney, Australia.
- KMID: 2471826
- DOI: http://doi.org/10.5534/wjmh.180099
Abstract
- PURPOSE
Patients with non-seminoma testicular cancer (NSTC) cancer can be subfertile or infertile, and present reduced sperm quality, but the underlying mechanisms are unknown. The aim of this study was to compare the sperm proteome of patients with NSTC, who cryopreserved their sperm before starting cancer treatment, with that from healthy fertile men.
MATERIALS AND METHODS
Semen volume, sperm motility and sperm concentration were evaluated before the cryopreservation of samples from patients with NSTC (n=15) and the control group (n=15). Sperm proteomic analysis was performed by liquid chromatography-tandem mass spectrometry and the differentially expressed proteins (DEPs) between the two groups were identified using bioinformatic tools.
RESULTS
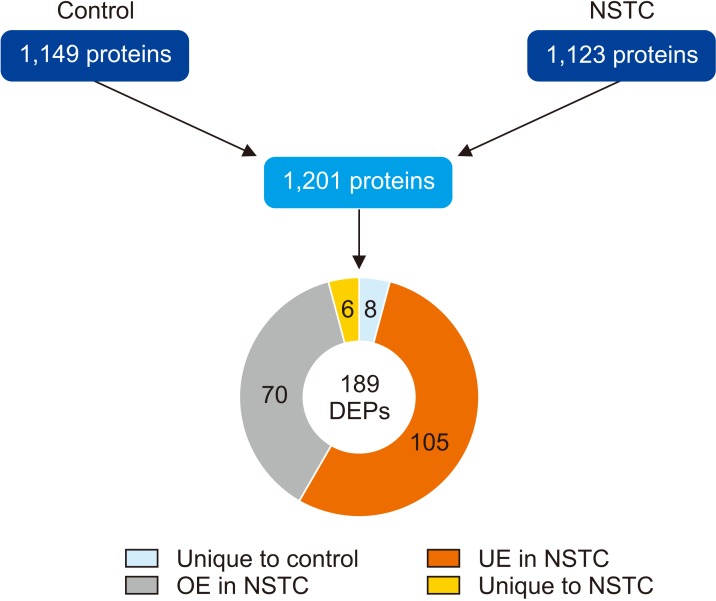
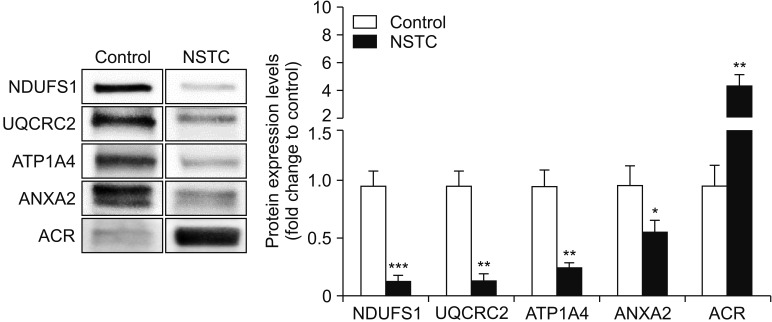
A total of 189 DEPs was identified in the dataset, from which five DEPs related to sperm function and fertilization were selected for validation by Western blot. We were able to validate the underexpression of the mitochondrial complex subunits NADH:Ubiquinone Oxidoreductase Core Subunit S1 (NDUFS1) and ubiquinol-cytochrome C reductase core protein 2 (UQCRC2), as well as the underexpression of the testis-specific sodium/potassium-transporting ATPase subunit alpha-4 (ATP1A4) in the NSTC group.
CONCLUSIONS
Our results indicate that sperm mitochondrial dysfunction may explain the observed decrease in sperm concentration, total sperm count and total motile count in NSTC patients. The identified DEPs may serve as potential biomarkers for the pathophysiology of subfertility/infertility in patients with NSTC. Our study also associates the reduced fertilizing ability of NSTC patients with the dysregulation of important sperm molecular mechanisms.
MeSH Terms
-
Adenosine Triphosphatases
Biomarkers
Blotting, Western
Computational Biology
Cryopreservation
Dataset
Electron Transport Complex III
Fertility*
Fertilization
Humans
Male
Mass Spectrometry
Proteome
Semen
Sperm Count
Sperm Motility
Spermatozoa
Testicular Neoplasms*
Adenosine Triphosphatases
Biomarkers
Electron Transport Complex III
Proteome
Figure
Reference
-
1. Manecksha RP, Fitzpatrick JM. Epidemiology of testicular cancer. BJU Int. 2009; 104:1329–1333. PMID: 19840008.
Article2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017; 67:7–30. PMID: 28055103.
Article3. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018; 68:7–30. PMID: 29313949.
Article4. Tvrda E, Agarwal A, Alkuhaimi N. Male reproductive cancers and infertility: a mutual relationship. Int J Mol Sci. 2015; 16:7230–7260. PMID: 25837470.
Article5. Garner MJ, Turner MC, Ghadirian P, Krewski D. Epidemiology of testicular cancer: an overview. Int J Cancer. 2005; 116:331–339. PMID: 15818625.
Article6. Oldenburg J, Fosså SD, Nuver J, Heidenreich A, Schmoll HJ, Bokemeyer C, et al. Testicular seminoma and non-seminoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013; 24(Suppl 6):vi125–vi132. PMID: 24078656.
Article7. Eble JN, Sauter G, Epstein JI, Sesterhenn IA. World Health Organization. International Agency for Research on Cancer. International Academy of Pathology. Pathology and genetics of tumours of the urinary system and male genital organs. Lyons: IARC Press;2004. p. 250–262.8. Noone AM, Howlader N, Krapcho M, Miller D, Brest A, Yu M, et al. SEER cancer statistics review (CSR), 1975-2015. Bethesda: National Cancer Institute;2009.9. Oosterhuis JW, Looijenga LH. Testicular germ-cell tumours in a broader perspective. Nat Rev Cancer. 2005; 5:210–222. PMID: 15738984.
Article10. Albers P, Albrecht W, Algaba F, Bokemeyer C, Cohn-Cedermark G, Horwich A, et al. Guidelines on testicular cancer. Eur Urol. 2005; 48:885–894. PMID: 16126333.
Article11. Howell SJ, Shalet SM. Spermatogenesis after cancer treatment: damage and recovery. J Natl Cancer Inst Monogr. 2005; 12–17.
Article12. Ping P, Gu BH, Li P, Huang YR, Li Z. Fertility outcome of patients with testicular tumor: before and after treatment. Asian J Androl. 2014; 16:107–111. PMID: 24369141.
Article13. Meistrich ML. Effects of chemotherapy and radiotherapy on spermatogenesis in humans. Fertil Steril. 2013; 100:1180–1186. PMID: 24012199.
Article14. Huyghe E, Matsuda T, Daudin M, Chevreau C, Bachaud JM, Plante P, et al. Fertility after testicular cancer treatments: results of a large multicenter study. Cancer. 2004; 100:732–737. PMID: 14770428.15. Agarwal A. Semen banking in patients with cancer: 20-year experience. Int J Androl. 2000; 23(Suppl 2):16–19. PMID: 10849486.
Article16. Spermon JR, Kiemeney LA, Meuleman EJ, Ramos L, Wetzels AM, Witjes JA. Fertility in men with testicular germ cell tumors. Fertil Steril. 2003; 79(Suppl 3):1543–1549. PMID: 12801557.
Article17. Smith ZL, Werntz RP, Eggener SE. Testicular cancer: epidemiology, diagnosis, and management. Med Clin North Am. 2018; 102:251–264. PMID: 29406056.18. Jacobsen R, Bostofte E, Engholm G, Hansen J, Olsen JH, Skakkebaek NE, et al. Risk of testicular cancer in men with abnormal semen characteristics: cohort study. BMJ. 2000; 321:789–792. PMID: 11009515.
Article19. Eisenberg ML, Li S, Brooks JD, Cullen MR, Baker LC. Increased risk of cancer in infertile men: analysis of U.S. claims data. J Urol. 2015; 193:1596–1601. PMID: 25463997.
Article20. Walsh TJ, Croughan MS, Schembri M, Chan JM, Turek PJ. Increased risk of testicular germ cell cancer among infertile men. Arch Intern Med. 2009; 169:351–356. PMID: 19237718.
Article21. Raman JD, Nobert CF, Goldstein M. Increased incidence of testicular cancer in men presenting with infertility and abnormal semen analysis. J Urol. 2005; 174:1819–1822. discussion 1822. PMID: 16217294.
Article22. Gandini L, Lombardo F, Salacone P, Paoli D, Anselmo AP, Culasso F, et al. Testicular cancer and Hodgkin’s disease: evaluation of semen quality. Hum Reprod. 2003; 18:796–801. PMID: 12660273.
Article23. Petersen PM, Skakkebaek NE, Vistisen K, Rørth M, Giwercman A. Semen quality and reproductive hormones before orchiectomy in men with testicular cancer. J Clin Oncol. 1999; 17:941–947. PMID: 10071288.
Article24. Morrish DW, Venner PM, Siy O, Barron G, Bhardwaj D, Outhet D. Mechanisms of endocrine dysfunction in patients with testicular cancer. J Natl Cancer Inst. 1990; 82:412–418. PMID: 2304089.
Article25. Agarwal A, Bertolla RP, Samanta L. Sperm proteomics: potential impact on male infertility treatment. Expert Rev Proteomics. 2016; 13:285–296. PMID: 26853600.
Article26. Sharma R, Agarwal A, Mohanty G, Du Plessis SS, Gopalan B, Willard B, et al. Proteomic analysis of seminal fluid from men exhibiting oxidative stress. Reprod Biol Endocrinol. 2013; 11:85. PMID: 24004880.
Article27. Intasqui P, Agarwal A, Sharma R, Samanta L, Bertolla RP. Towards the identification of reliable sperm biomarkers for male infertility: a sperm proteomic approach. Andrologia. 2018; 50.
Article28. Baker MA. Proteomics of post-translational modifications of mammalian spermatozoa. Cell Tissue Res. 2016; 363:279–287. PMID: 26239910.
Article29. World Health Organization. WHO laboratory manual for the examination and processing of human semen. 5th ed. Geneva: World Health Organization;2010.30. Agarwal AS, Gupta S, Sharma R. Cryopreservation of client depositor semen. In : Agarwal AS, Gupta S, Sharma R, editors. Andrological evaluation of male infertility: a laboratory guide. Switzerland: Springer;2016. p. 111–133.31. Martínez-Bartolomé S, Deutsch EW, Binz PA, Jones AR, Eisenacher M, Mayer G, et al. Guidelines for reporting quantitative mass spectrometry based experiments in proteomics. J Proteomics. 2013; 95:84–88. PMID: 23500130.
Article32. Agarwal A, Ayaz A, Samanta L, Sharma R, Assidi M, Abuzenadah AM, et al. Comparative proteomic network signatures in seminal plasma of infertile men as a function of reactive oxygen species. Clin Proteomics. 2015; 12:23. PMID: 26321892.
Article33. Agarwal AS, Gupta S, Sharma R. Reactive oxygen species (ROS) measurement. In : Agarwal AS, Gupta S, Sharma R, editors. Andrological evaluation of male infertility: a laboratory guide. Switzerland: Springer;2016. p. 155–163.34. Practice Committee of the American Society for Reproductive Medicine. Effectiveness and treatment for unexplained infertility. Fertil Steril. 2006; 86:S111–S114. PMID: 17055802.35. Agarwal A, Burns WR, Dada R, Sabanegh ES. Male infertility and testicular cancer-points of common causality. Euro Urol Rev. 2010; 5:56–59.36. Ferramosca A, Zara V. Mitochondria and fertility: the mitochondria critical role on spermatozoa function. JDREAM. 2017; 1:21–26.37. Aitken RJ. Reactive oxygen species as mediators of sperm capacitation and pathological damage. Mol Reprod Dev. 2017; 84:1039–1052. PMID: 28749007.
Article38. Cassina A, Silveira P, Cantu L, Montes JM, Radi R, Sapiro R. Defective human sperm cells are associated with mitochondrial dysfunction and oxidant production. Biol Reprod. 2015; 93:119. PMID: 26447142.
Article39. Mimaki M, Wang X, McKenzie M, Thorburn DR, Ryan MT. Understanding mitochondrial complex I assembly in health and disease. Biochim Biophys Acta. 2012; 1817:851–862. PMID: 21924235.
Article40. Janssen RJ, Nijtmans LG, van den Heuvel LP, Smeitink JA. Mitochondrial complex I: structure, function and pathology. J Inherit Metab Dis. 2006; 29:499–515. PMID: 16838076.
Article41. Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009; 417:1–13. PMID: 19061483.
Article42. Shukla KK, Kwon WS, Rahman MS, Park YJ, You YA, Pang MG. Nutlin-3a decreases male fertility via UQCRC2. PLoS One. 2013; 8:e76959. PMID: 24130818.
Article43. de Lamirande E, O’Flaherty C. Sperm activation: role of reactive oxygen species and kinases. Biochim Biophys Acta. 2008; 1784:106–115. PMID: 17920343.
Article44. Samanta L, Agarwal A, Swain N, Sharma R, Gopalan B, Esteves SC, et al. Proteomic signatures of sperm mitochondria in varicocele: clinical utility as biomarkers of varicocele associated infertility. J Urol. 2018; 200:414–422. PMID: 29530785.45. Pasqualotto FF, Sharma RK, Nelson DR, Thomas AJ, Agarwal A. Relationship between oxidative stress, semen characteristics, and clinical diagnosis in men undergoing infertility investigation. Fertil Steril. 2000; 73:459–464. PMID: 10688996.
Article46. Peña FJ, Rodríguez Martínez H, Tapia JA, Ortega Ferrusola C, González Fernández L, Macías García B. Mitochondria in mammalian sperm physiology and pathology: a review. Reprod Domest Anim. 2009; 44:345–349.
Article47. Saxton RA, Sabatini DM. mTOR signaling in growth, metabolism, and disease. Cell. 2017; 168:960–976. PMID: 28283069.
Article48. Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012; 149:274–293. PMID: 22500797.
Article49. Dong H, Chen Z, Wang C, Xiong Z, Zhao W, Jia C, et al. Rictor regulates spermatogenesis by controlling sertoli cell cytoskeletal organization and cell polarity in the mouse testis. Endocrinology. 2015; 156:4244–4256. PMID: 26360620.
Article50. Oliveira PF, Cheng CY, Alves MG. Emerging role for Mammalian target of rapamycin in male fertility. Trends Endocrinol Metab. 2017; 28:165–167. PMID: 28063768.
Article51. Mok KW, Mruk DD, Lee WM, Cheng CY. Rictor/mTORC2 regulates blood-testis barrier dynamics via its effects on gap junction communications and actin filament network. FASEB J. 2013; 27:1137–1152. PMID: 23288930.52. Xu K, Liu P, Wei W. mTOR signaling in tumorigenesis. Biochim Biophys Acta. 2014; 1846:638–654. PMID: 25450580.
Article53. Baker MJ, Lampe PA, Stojanovski D, Korwitz A, Anand R, Tatsuta T, et al. Stress‐induced OMA1 activation and autocatalytic turnover regulate OPA1‐dependent mitochondrial dynamics. EMBO J. 2014; 33:578–593. PMID: 24550258.
Article54. Quirós PM, Ramsay AJ, Sala D, Fernández-Vizarra E, Rodríguez F, Peinado JR, et al. Loss of mitochondrial protease OMA1 alters processing of the GTPase OPA1 and causes obesity and defective thermogenesis in mice. EMBO J. 2012; 31:2117–2133. PMID: 22433842.
Article55. Jimenez T, McDermott JP, Sánchez G, Blanco G. Na,KATPase alpha4 isoform is essential for sperm fertility. Proc Natl Acad Sci U S A. 2011; 108:644–649. PMID: 21187400.56. Hlivko JT, Chakraborty S, Hlivko TJ, Sengupta A, James PF. The human Na,K‐ATPase alpha4 isoform is a ouabain‐sensitive alpha isoform that is expressed in sperm. Mol Reprod Dev. 2006; 73:101–115. PMID: 16175638.
Article57. Koçak-Toker N, Aktan G, Aykaç-Toker G. The role of Na,K-ATPase in human sperm motility. Int J Androl. 2002; 25:180–185. PMID: 12031047.
Article58. Sanchez G, Nguyen AN, Timmerberg B, Tash JS, Blanco G. The Na,K-ATPase alpha4 isoform from humans has distinct enzymatic properties and is important for sperm motility. Mol Hum Reprod. 2006; 12:565–576. PMID: 16861705.59. Bharadwaj A, Bydoun M, Holloway R, Waisman D. Annexin A2 heterotetramer: structure and function. Int J Mol Sci. 2013; 14:6259–6305. PMID: 23519104.
Article60. Burden HP, Holmes CH, Persad R, Whittington K. Prostasomes: their effects on human male reproduction and fertility. Hum Reprod Update. 2006; 12:283–292. PMID: 16373403.61. Feinberg JM, Rainteau DP, Kaetzel MA, Dacheux JL, Dedman JR, Weinman SJ. Differential localization of annexins in ram germ cells: a biochemical and immunocytochemical study. J Histochem Cytochem. 1991; 39:955–963. PMID: 1830893.
Article62. Palmerini CA, Carlini E, Nicolucci A, Arienti G. Increase of human spermatozoa intracellular Ca2+ concentration after fusion with prostasomes. Cell Calcium. 1999; 25:291–296. PMID: 10456226.63. Ho HC, Suarez SS. Characterization of the intracellular calcium store at the base of the sperm flagellum that regulates hyperactivated motility. Biol Reprod. 2003; 68:1590–1596. PMID: 12606347.