Korean J Radiol.
2020 Apr;21(4):483-493. 10.3348/kjr.2019.0739.
Use of Magnetic Resonance Neurography for Evaluating the Distribution and Patterns of Chronic Inflammatory Demyelinating Polyneuropathy
- Affiliations
-
- 1Department of Radiology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China. whxhchuansheng@126.com
- 2Hubei Province Key Laboratory of Molecular Imaging, Wuhan, China.
- 3Department of Neurology, Renming Hospital of Wuhan University, Wuhan, China.
- 4MR Scientific Marketing, Siemens Healthineers, Shanghai, China.
- KMID: 2471814
- DOI: http://doi.org/10.3348/kjr.2019.0739
Abstract
OBJECTIVE
To evaluate the distribution and characteristics of peripheral nerve abnormalities in chronic inflammatory demyelinating polyneuropathy (CIDP) using magnetic resonance neurography (MRN) and to examine the diagnostic efficiency.
MATERIALS AND METHODS
Thirty-one CIDP patients and 21 controls underwent MR scans. Three-dimensional sampling perfections with application-optimized contrasts using different flip-angle evolutions and T1-/T2- weighted turbo spin-echo sequences were performed for neurography of the brachial and lumbosacral (LS) plexus and cauda equina, respectively. Clinical data and scores of the inflammatory Rasch-built overall disability scale (I-RODS) in CIDP were obtained.
RESULTS
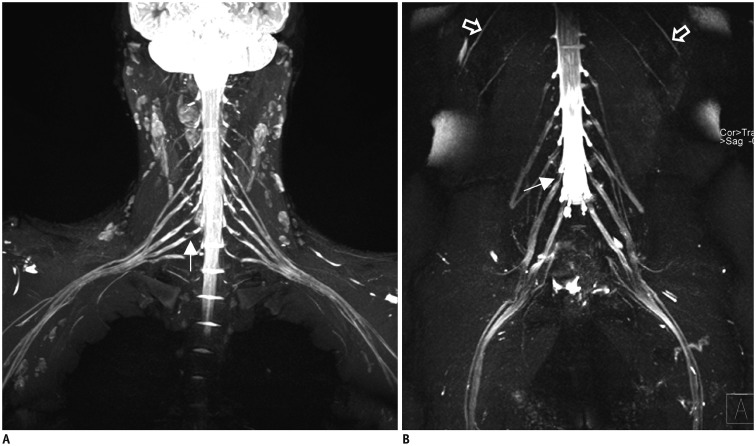
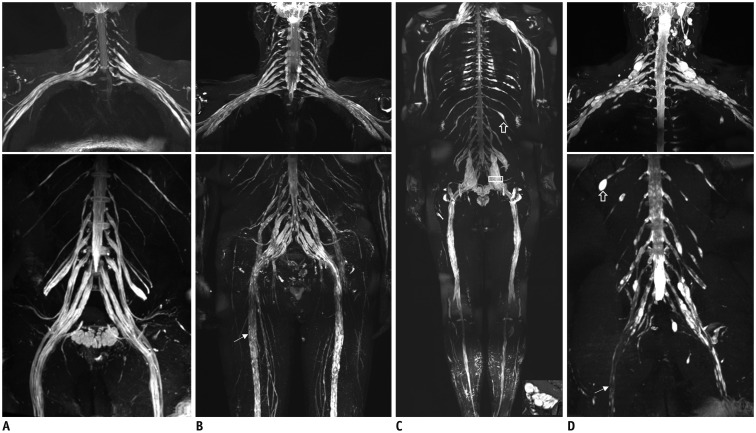
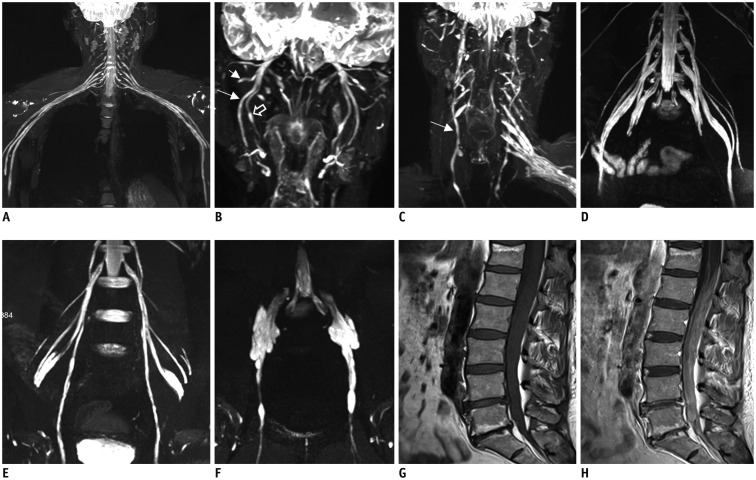
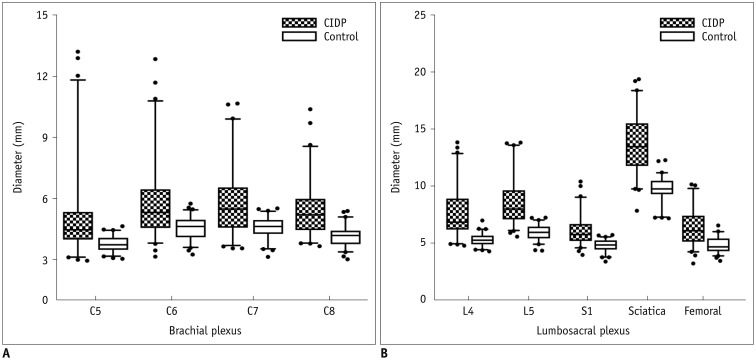
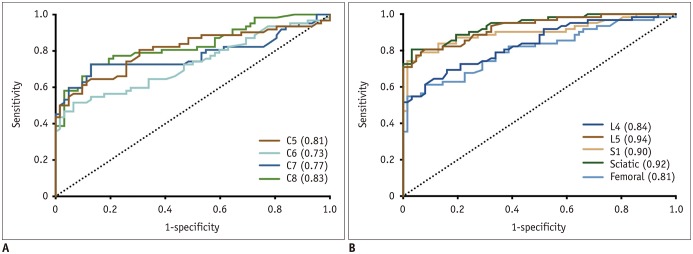
The bilateral extracranial vagus (n = 11), trigeminal (n = 12), and intercostal nerves (n = 10) were hypertrophic. Plexus hypertrophies were observed in the brachial plexus of 19 patients (61.3%) and in the LS plexus of 25 patients (80.6%). Patterns of hypertrophy included uniform hypertrophy (17 [54.8%] brachial plexuses and 21 [67.7%] LS plexuses), and multifocal fusiform hypertrophy (2 [6.5%] brachial plexuses and 4 [12.9%] LS plexuses) was present. Enlarged and/or contrast-enhanced cauda equina was found in 3 (9.7%) and 13 (41.9%) patients, respectively. Diameters of the brachial and LS nerve roots were significantly larger in CIDP than in controls (p < 0.001). The largest AUC was obtained for the L5 nerve. There were no significant differences in the course duration, I-RODS score, or diameter between patients with and without hypertrophy.
CONCLUSION
MRN is useful for the assessment of distribution and characteristics of the peripheral nerves in CIDP. Compared to other regions, LS plexus neurography is more sensitive for CIDP.
Keyword
MeSH Terms
Figure
Reference
-
1. Vallat JM, Sommer C, Magy L. Chronic inflammatory demyelinating polyradiculoneuropathy: diagnostic and therapeutic challenges for a treatable condition. Lancet Neurol. 2010; 9:402–412. PMID: 20298964.
Article2. Rotta FT, Sussman AT, Bradley WG, Ram Ayyar D, Sharma KR, Shebert RT. The spectrum of chronic inflammatory demyelinating polyneuropathy. J Neurol Sci. 2000; 173:129–139. PMID: 10675657.
Article3. Alabdali M, Abraham A, Alsulaiman A, Breiner A, Barnett C, Katzberg HD, et al. Clinical characteristics, and impairment and disability scale scores for different CIDP disease activity status classes. J Neurol Sci. 2017; 372:223–227. PMID: 28017218.
Article4. de Silva RN, Willison HJ, Doyle D, Weir AI, Hadley DM, Thomas AM. Nerve root hypertrophy in chronic inflammatory demyelinating polyneuropathy. Muscle Nerve. 1994; 17:168–170. PMID: 8114785.
Article5. Iijima M, Koike H, Hattori N, Tamakoshi A, Katsuno M, Tanaka F, et al. Prevalence and incidence rates of chronic inflammatory demyelinating polyneuropathy in the Japanese population. J Neurol Neurosurg Psychiatry. 2008; 79:1040–1043. PMID: 18223015.
Article6. Rajabally YA, Stettner M, Kieseier BC, Hartung HP, Malik RA. CIDP and other inflammatory neuropathies in diabetes - Diagnosis and management. Nat Rev Neurol. 2017; 13:599–611. PMID: 28914883.
Article7. Latov N. Diagnosis and treatment of chronic acquired demyelinating polyneuropathies. Nat Rev Neurol. 2014; 10:435–446. PMID: 24980070.
Article8. Scheidl E, Böhm J, Simó M, Rózsa C, Bereznai B, Kovács T, et al. Ultrasonography of MADSAM neuropathy: focal nerve enlargements at sites of existing and resolved conduction blocks. Neuromuscul Disord. 2012; 22:627–631. PMID: 22513319.
Article9. Mathey EK, Park SB, Hughes RA, Pollard JD, Armati PJ, Barnett MH, et al. Chronic inflammatory demyelinating polyradiculoneuropathy: from pathology to phenotype. J Neurol Neurosurg Psychiatry. 2015; 86:973–985. PMID: 25677463.
Article10. Van den Bergh PY, Hadden RD, Bouche P, Cornblath DR, Hahn A, Illa I, et al. European Federation of Neurological Societies/Peripheral Nerve Society guideline on management of chronic inflammatory demyelinating polyradiculoneuropathy: report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society - First revision. Eur J Neurol. 2010; 17:356–363. PMID: 20456730.11. Bradley LJ, Wilhelm T, King RH, Ginsberg L, Orrell RW. Brachial plexus hypertrophy in chronic inflammatory demyelinating polyradiculoneuropathy. Neuromuscul Disord. 2006; 16:126–131. PMID: 16427285.
Article12. Hiwatashi A, Togao O, Yamashita K, Kikuchi K, Ogata H, Yamasaki R, et al. Evaluation of chronic inflammatory demyelinating polyneuropathy: 3D nerve-sheath signal increased with inked rest-tissue rapid acquisition of relaxation enhancement imaging (3D SHINKEI). Eur Radiol. 2017; 27:447–453. PMID: 27208993.
Article13. Mürtz P, Kaschner M, Lakghomi A, Gieseke J, Willinek WA, Schild HH, et al. Diffusion-weighted MR neurography of the brachial and lumbosacral plexus: 3.0 T versus 1.5 T imaging. Eur J Radiol. 2015; 84:696–702. PMID: 25623827.14. Lee JH, Cheng KL, Choi YJ, Baek JH. High-resolution imaging of neural anatomy and pathology of the neck. Korean J Radiol. 2017; 18:180–193. PMID: 28096728.
Article15. Wang L, Niu Y, Kong X, Yu Q, Kong X, Lv Y, et al. The application of paramagnetic contrast-based T2 effect to 3D heavily T2W high-resolution MR imaging of the brachial plexus and its branches. Eur J Radiol. 2016; 85:578–584. PMID: 26860670.
Article16. van Nes SI, Vanhoutte EK, van Doorn PA, Hermans M, Bakkers M, Kuitwaard K, et al. Rasch-built overall disability scale (R-ODS) for immune-mediated peripheral neuropathies. Neurology. 2011; 76:337–345. PMID: 21263135.
Article17. Shibuya K, Sugiyama A, Ito S, Misawa S, Sekiguchi Y, Mitsuma S, et al. Reconstruction magnetic resonance neurography in chronic inflammatory demyelinating polyneuropathy. Ann Neurol. 2015; 77:333–337. PMID: 25425460.
Article18. Grothues F, Smith GC, Moon JC, Bellenger NG, Collins P, Klein HU, et al. Comparison of interstudy reproducibility of cardiovascular magnetic resonance with two-dimensional echocardiography in normal subjects and in patients with heart failure or left ventricular hypertrophy. Am J Cardiol. 2002; 90:29–34. PMID: 12088775.
Article19. Kim SK, Jeong MY, Kang HK, Yoon W. Diffusion-weighted magnetic resonance imaging findings in a patient with trigeminal ganglioneuroma. Korean J Radiol. 2013; 14:118–121. PMID: 23323041.
Article20. Behzadi AH, Farooq Z, Zhao Y, Shih G, Prince MR. Dentate nucleus signal intensity decrease on T1-weighted MR images after switching from gadopentetate dimeglumine to gadobutrol. Radiology. 2018; 287:816–823. PMID: 29533723.21. Duarte J, Martinez AC, Rodriguez F, Mendoza A, Sempere AP, Claveria LE. Hypertrophy of multiple cranial nerves and spinal roots in chronic inflammatory demyelinating neuropathy. J Neurol Neurosurg Psychiatry. 1999; 67:685–687. PMID: 10519883.
Article22. Zhang Z, Meng Q, Chen Y, Li Z, Luo B, Yang Z, et al. 3-T imaging of the cranial nerves using three-dimensional reversed FISP with diffusion-weighted MR sequence. J Magn Reson Imaging. 2008; 27:454–458. PMID: 18219629.
Article23. Oguz B, Oguz KK, Cila A, Tan E. Diffuse spinal and intercostal nerve involvement in chronic inflammatory demyelinating polyradiculoneuropathy: MRI findings. Eur Radiol. 2003; 13:L230–L234.
Article24. Matsuda M, Ikeda S, Sakurai S, Nezu A, Yanagisawa N, Inuzuka T. Hypertrophic neuritis due to chronic inflammatory demyelinating polyradiculoneuropathy (CIDP): a postmortem pathological study. Muscle Nerve. 1996; 19:163–169. PMID: 8559164.
Article25. Arvidson B. A study of the perineurial diffusion barrier of a peripheral ganglion. Acta Neuropathol. 1979; 46:139–144. PMID: 452854.
Article26. Jacobs JM, Macfarlane RM, Cavanagh JB. Vascular leakage in the dorsal root ganglia of the rat, studied with horseradish peroxidase. J Neurol Sci. 1976; 29:95–107. PMID: 950578.
Article27. Olsson Y, Reese TS. Permeability of vasa nervorum and perineurium in mouse sciatic nerve studied by fluorescence and electron microscopy. J Neuropathol Exp Neurol. 1971; 30:105–119. PMID: 5542492.
Article28. Shimizu F, Sawai S, Sano Y, Beppu M, Misawa S, Nishihara H, et al. Severity and patterns of blood-nerve barrier breakdown in patients with chronic inflammatory demyelinating polyradiculoneuropathy: correlations with clinical subtypes. PLoS One. 2014; 9:e104205. PMID: 25105500.
Article29. Kanda T, Numata Y, Mizusawa H. Chronic inflammatory demyelinating polyneuropathy: decreased claudin-5 and relocated ZO-1. J Neurol Neurosurg Psychiatry. 2004; 75:765–769. PMID: 15090575.
Article30. Duggins AJ, McLeod JG, Pollard JD, Davies L, Yang F, Thompson EO, et al. Spinal root and plexus hypertrophy in chronic inflammatory demyelinating polyneuropathy. Brain. 1999; 122:1383–1390. PMID: 10388803.
Article31. Eurelings M, Notermans NC, Franssen H, Van Es HW, Ramos LM, Wokke JH, et al. MRI of the brachial plexus in polyneuropathy associated with monoclonal gammopathy. Muscle Nerve. 2001; 24:1312–1318. PMID: 11562910.
Article32. Härtig F, Ross M, Dammeier NM, Fedtke N, Heiling B, Axer H, et al. Nerve ultrasound predicts treatment response in chronic inflammatory demyelinating polyradiculoneuropathy-a prospective follow-up. Neurotherapeutics. 2018; 15:439–451. PMID: 29435815.
Article33. Jongbloed BA, Bos JW, Rutgers D, van der Pol WL, van den Berg LH. Brachial plexus magnetic resonance imaging differentiates between inflammatory neuropathies and does not predict disease course. Brain Behav. 2017; 7:e00632. PMID: 28523213.
Article34. Akcar N, Ozkan S, Mehmetoglu O, Calisir C, Adapinar B. Value of power Doppler and gray-scale US in the diagnosis of carpal tunnel syndrome: contribution of cross-sectional area just before the tunnel inlet as compared with the cross-sectional area at the tunnel. Korean J Radiol. 2010; 11:632–639. PMID: 21076589.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Interpretation of Electrodiagnostic Tests in Chronic Inflammatory Demyelinating Polyneuropathy: Classification Using Nerve Conduction Study
- Subacute Inflammatory Demyelinating Polyneuropathy Combined with Optic Neuritis
- Delayed Relaxation (Pseudomyotonia) as the Only Clinical Manifestation of Chronic Inflammatory Demyelinating Polyneuropathy
- Chronic Inflammatory Demyelinating Polyneuropathy Combined With Limited Cutaneous Systemic Sclerosis
- Electrophysiological and radiological evidence for the multifocal nature of a case of multifocal acquired demyelinating sensory and motor neuropathy