J Korean Ophthalmol Soc.
2020 Mar;61(3):258-266. 10.3341/jkos.2020.61.3.258.
Comparison of Clinical Characteristics between Patients with Cytomegalovirus Positive and Negative Hypertensive Uveitis
- Affiliations
-
- 1Department of Ophthalmology, College of Medicine, The Catholic University, Seoul, Korea. jinah616@hanmail.net
- KMID: 2471766
- DOI: http://doi.org/10.3341/jkos.2020.61.3.258
Abstract
- PURPOSE
To compare the clinical characteristics of hypertensive uveitis patients, with and without cytomegalovirus (CMV) infection.
METHODS
CMV polymerase chain reaction (PCR) was performed on the aqueous humor of 61 patients with hypertensive uveitis. Patients were divided into CMV positive and negative groups. Sex, age, age at first attack and at first diagnosis, duration of attack, number of attacks, interval between attacks, and surgical history were investigated, and the visual acuity, intraocular pressure (IOP), and corneal endothelial cell density were measured. Blood tests were conducted to determine the inflammation index, antibody titers of CMV, and herpes simplex virus, with toxoplasma and toxocariasis evaluations. With these results, the differences between the two groups were confirmed.
RESULTS
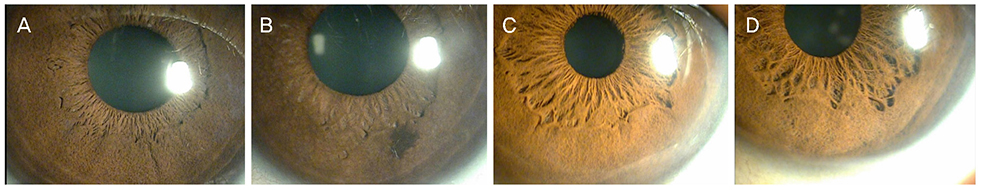
Compared with the CMV negative group, the CMV positive group showed a significantly higher trough IOP (p = 0.007) and a greater difference in corneal endothelial cell counts of the affected eye and the fellow eye (p = 0.048). The CMV positive group exhibited iris degeneration (73.3%), whereas the CMV negative group showed lesions in 47.8% (p = 0.085). No statistically significant differences between the two groups were evident in terms of leukocyte count, inflammation index, antibody titers to CMV, HSV, toxoplasma, or toxocariasis.
CONCLUSIONS
CMV anterior uveitis was characterized by high IOP and corneal endothelial cell loss in the affected eyes. The CMV positive group had more corneal lesions and iris degeneration than the CMV negative group; however, the two groups showed no significant serological differences. When the clinical features of hypertensive uveitis are present, a routine corneal endothelium test and CMV PCR should be performed periodically, to initiate antiviral agent treatments along with IOP and inflammation controls.
MeSH Terms
Figure
Reference
-
1. Markomichelakis NN, Canakis C, Zafirakis P, et al. Cytomegalovirus as a cause of anterior uveitis with sectoral iris atrophy. Ophthalmology. 2002; 109:879–882.
Article2. Chan NS, Chee SP. Demystifying viral anterior uveitis: a review. Clin Exp Ophthalmol. 2019; 47:320–333.
Article3. Yoser SL, Forster DJ, Rao NA. Systemic viral infections and their retinal and choroidal manifestations. Surv Ophthalmol. 1993; 37:313–352.
Article4. Touhami S, Qu L, Angi M, et al. Cytomegalovirus anterior uveitis: clinical characteristics and long-term outcomes in a French Series. Am J Ophthalmol. 2018; 194:134–142.
Article5. Knox DL. Clinical features of cytomegalovirus anterior uveitis in immunocompetent patients. Am J Ophthalmol. 2008; 146:625.
Article6. Su CC, Hu FR, Wang TH, et al. Clinical outcomes in cytomegalovirus-positive Posner-Schlossman syndrome patients treated with topical ganciclovir therapy. Am J Ophthalmol. 2014; 158:1024–1031.e2.
Article7. Kass MA, Becker B, Kolker AE. Glaucomatocyclitic crisis and primary open-angle glaucoma. Am J Ophthalmol. 1973; 75:668–673.
Article8. Jabs DA, Nussenblatt RB, Rosenbaum JT. Standardization of Uveitis Nomenclature (SUN) Working Group. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol. 2005; 140:509–516.9. Cunningham ET Jr, Zierhut M. Uveitic ocular hypertension and glaucoma. Ocul Immunol Inflamm. 2017; 25:737–739.
Article10. Lee EJ, Kwun YK, Shin DH, Kee CW. Clinical features and risk factors of glaucomatous change in Posner-Schlossman Syndrome. J Korean Ophthalmol Soc. 2015; 56:938–943.
Article11. Lo CY, Ho KN, Yuen KY, et al. Diagnosing cytomegalovirus disease in CMV seropositive renal allograft recipients: a comparison between the detection of CMV DNAemia by polymerase chain reaction and antigenemia by CMV pp65 assay. Clin Transplant. 1997; 11:286–293.12. Chee SP, Jap A. Cytomegalovirus anterior uveitis: outcome of treatment. Br J Ophthalmol. 2010; 94:1648–1652.
Article13. Su CC, Wang IJ, Chen WL, et al. Topical ganciclovir treatment in patients with cytomegalovirus endotheliitis receiving penetrating keratoplasty. Clin Exp Ophthalmol. 2013; 41:339–347.
Article14. Chan NS, Chee SP, Caspers L, Bodaghi B. Clinical features of CMV-associated anterior uveitis. Ocul Immunol Inflamm. 2018; 26:107–115.
Article15. Relvas LJ, Caspers L, Chee SP, et al. Differential diagnosis of viralinduced anterior uveitis. Ocul Immunol Inflamm. 2018; 26:726–731.
Article16. Chee SP, Bacsal K, Jap A, et al. Clinical features of cytomegalovirus anterior uveitis in immunocompetent patients. Am J Ophthalmol. 2008; 145:834–840.
Article17. Hwang YS, Shen CR, Chang SH, et al. The validity of clinical feature profiles for cytomegaloviral anterior segment infection. Graefes Arch Clin Exp Ophthalmol. 2011; 249:103–110.
Article18. Kandori M, Miyazaki D, Yakura K, et al. Relationship between the number of cytomegalovirus in anterior chamber and severity of anterior segment inflammation. Jpn J Ophthalmol. 2013; 57:497–502.
Article19. Choi JA, Kim KS, Jung Y, et al. Cytomegalovirus as a cause of hypertensive anterior uveitis in immunocompetent patients. J Ophthalmic Inflamm Infect. 2016; 6:32.
Article20. De Groot-Mijnes JDF, Chan ASY, Chee SP, Verjans GMGM. Immunopathology of virus-induced anterior uveitis. Ocul Immunol Inflamm. 2018; 26:338–346.
Article21. Hayashi K, Kurihara I, Uchida Y. Studies of ocular murine cytomegalovirus infection. Invest Ophthalmol Vis Sci. 1985; 26:486–493.22. Bale JF Jr, O'Neil ME, Lyon B, Perlman S. The pathogenesis of murine cytomegalovirus ocular infection. Anterior chamber inoculation. Invest Ophthalmol Vis Sci. 1990; 31:1575–1581.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cytomegalovirus as a Cause of Recurrent Anterior Uveitis in Immunocompetent Patients
- Clinical Analysis of Uveitis by the Diagnostic Studies
- Cytomegalovirus retinitis with panretinal occlusive vasculopathy concealed by hypertensive uveitis: a case report
- Clinical Features of HLA-B27 Positive and Negative Acute Anterior Uveitis
- Uveitis in Infancy