Endocrinol Metab.
2020 Mar;35(1):36-43. 10.3803/EnM.2020.35.1.36.
Multifaceted Actions of Succinate as a Signaling Transmitter Vary with Its Cellular Locations
- Affiliations
-
- 1Department of Basic Science and Craniofacial Biology, New York University College of Dentistry, New York, NY, USA. xl15@nyu.edu
- 2Department of Internal Medicine, Seoul National University Hospital, Seoul, Korea.
- 3Perlmutter Cancer Institute, New York University Grossman School of Medicine, New York, NY, USA.
- 4Department of Urology, New York University Grossman School of Medicine, New York, NY, USA.
- KMID: 2471718
- DOI: http://doi.org/10.3803/EnM.2020.35.1.36
Abstract
- Since the identification of succinate's receptor in 2004, studies supporting the involvement of succinate signaling through its receptor in various diseases have accumulated and most of these investigations have highlighted succinate's pro-inflammatory role. Taken with the fact that succinate is an intermediate metabolite in the center of mitochondrial activity, and considering its potential regulation of protein succinylation through succinyl-coenzyme A, a review on the overall multifaceted actions of succinate to discuss whether and how these actions relate to the cellular locations of succinate is much warranted. Mechanistically, it is important to consider the sources of succinate, which include somatic cellular released succinate and those produced by the microbiome, especially the gut microbiota, which is an equivalent, if not greater contributor of succinate levels in the body. Continue learning the critical roles of succinate signaling, known and unknown, in many pathophysiological conditions is important. Furthermore, studies to delineate the regulation of succinate levels and to determine how succinate elicits various types of signaling in a temporal and spatial manner are also required.
Keyword
MeSH Terms
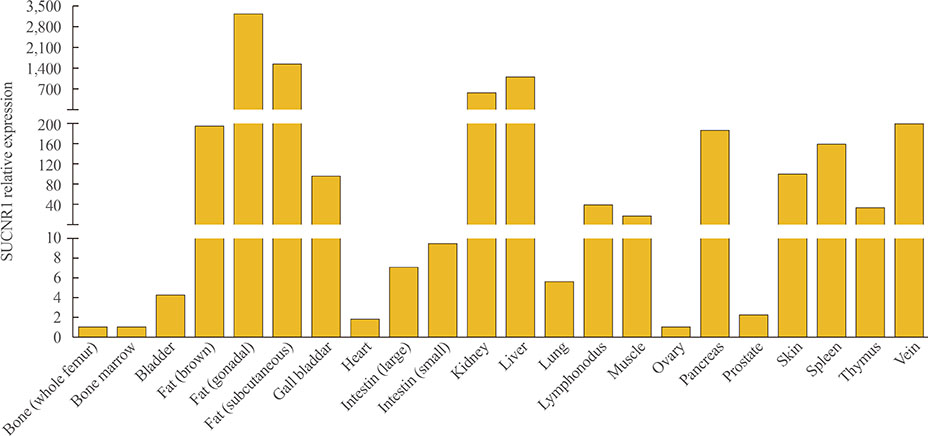
Figure
Cited by 1 articles
-
Gemigliptin Alleviates Succinate-Induced Hepatic Stellate Cell Activation by Ameliorating Mitochondrial Dysfunction
Giang Nguyen, So Young Park, Dinh Vinh Do, Dae-Hee Choi, Eun-Hee Cho
Endocrinol Metab. 2022;37(6):918-928. doi: 10.3803/EnM.2022.1530.
Reference
-
1. Chouchani ET, Pell VR, Gaude E, Aksentijevic D, Sundier SY, Robb EL, et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature. 2014; 515:431–435.
Article2. Lewis GD, Farrell L, Wood MJ, Martinovic M, Arany Z, Rowe GC, et al. Metabolic signatures of exercise in human plasma. Sci Transl Med. 2010; 2:33ra37.
Article3. Krebs HA. Rate control of the tricarboxylic acid cycle. Adv Enzyme Regul. 1970; 8:335–353.
Article4. Cecchini G. Function and structure of complex II of the respiratory chain. Annu Rev Biochem. 2003; 72:77–109.
Article5. Ackrell BA. Progress in understanding structure-function relationships in respiratory chain complex II. FEBS Lett. 2000; 466:1–5.
Article6. Lane M, Gardner DK. Mitochondrial malate-aspartate shuttle regulates mouse embryo nutrient consumption. J Biol Chem. 2005; 280:18361–18367.
Article7. Lowenstein JM. Ammonia production in muscle and other tissues: the purine nucleotide cycle. Physiol Rev. 1972; 52:382–414.
Article8. Haas R, Cucchi D, Smith J, Pucino V, Macdougall CE, Mauro C. Intermediates of metabolism: from bystanders to signalling molecules. Trends Biochem Sci. 2016; 41:460–471.
Article9. Connors J, Dawe N, Van Limbergen J. The role of succinate in the regulation of intestinal inflammation. Nutrients. 2019; 11:25.
Article10. Ferreyra JA, Wu KJ, Hryckowian AJ, Bouley DM, Weimer BC, Sonnenburg JL. Gut microbiota-produced succinate promotes C. difficile infection after antibiotic treatment or motility disturbance. Cell Host Microbe. 2014; 16:770–777.11. Gao YD, Zhao Y, Huang J. Metabolic modeling of common Escherichia coli strains in human gut microbiome. Biomed Res Int. 2014; 2014:694967.12. Li M, Gu D, Xu N, Lei F, Du L, Zhang Y, et al. Gut carbohydrate metabolism instead of fat metabolism regulated by gut microbes mediates high-fat diet-induced obesity. Benef Microbes. 2014; 5:335–344.
Article13. Macfarlane S, Macfarlane GT. Regulation of short-chain fatty acid production. Proc Nutr Soc. 2003; 62:67–72.
Article14. Leite AZ, Rodrigues NC, Gonzaga MI, Paiolo JCC, de Souza CA, Stefanutto NAV, et al. Detection of increased plasma interleukin-6 levels and prevalence of Prevotella copri and Bacteroides vulgatus in the feces of type 2 diabetes patients. Front Immunol. 2017; 8:1107.
Article15. Serena C, Ceperuelo-Mallafre V, Keiran N, Queipo-Ortuno MI, Bernal R, Gomez-Huelgas R, et al. Elevated circulating levels of succinate in human obesity are linked to specific gut microbiota. ISME J. 2018; 12:1642–1657.
Article16. Agani FH, Pichiule P, Chavez JC, LaManna JC. The role of mitochondria in the regulation of hypoxia-inducible factor 1 expression during hypoxia. J Biol Chem. 2000; 275:35863–35867.
Article17. Piantadosi CA, Suliman HB. Transcriptional regulation of SDHa flavoprotein by nuclear respiratory factor-1 prevents pseudo-hypoxia in aerobic cardiac cells. J Biol Chem. 2008; 283:10967–10977.
Article18. Tannahill GM, Curtis AM, Adamik J, Palsson-McDermott EM, McGettrick AF, Goel G, et al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature. 2013; 496:238–242.
Article19. Mills EL, Kelly B, Logan A, Costa ASH, Varma M, Bryant CE, et al. Succinate dehydrogenase supports metabolic repurposing of mitochondria to drive inflammatory macrophages. Cell. 2016; 167:457–470.
Article20. Mills EL, Pierce KA, Jedrychowski MP, Garrity R, Winther S, Vidoni S, et al. Accumulation of succinate controls activation of adipose tissue thermogenesis. Nature. 2018; 560:102–106.
Article21. Schwartz MW, Woods SC, Porte D Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000; 404:661–671.
Article22. Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006; 443:289–295.
Article23. Park J, Chen Y, Tishkoff DX, Peng C, Tan M, Dai L, et al. SIRT5-mediated lysine desuccinylation impacts diverse metabolic pathways. Mol Cell. 2013; 50:919–930.
Article24. Lin H, Su X, He B. Protein lysine acylation and cysteine succination by intermediates of energy metabolism. ACS Chem Biol. 2012; 7:947–960.
Article25. Gibson GE, Xu H, Chen HL, Chen W, Denton TT, Zhang S. Alpha-ketoglutarate dehydrogenase complex-dependent succinylation of proteins in neurons and neuronal cell lines. J Neurochem. 2015; 134:86–96.
Article26. Weinert BT, Scholz C, Wagner SA, Iesmantavicius V, Su D, Daniel JA, et al. Lysine succinylation is a frequently occurring modification in prokaryotes and eukaryotes and extensively overlaps with acetylation. Cell Rep. 2013; 4:842–851.
Article27. Zhang Z, Tan M, Xie Z, Dai L, Chen Y, Zhao Y. Identification of lysine succinylation as a new post-translational modification. Nat Chem Biol. 2011; 7:58–63.
Article28. Cimen H, Han MJ, Yang Y, Tong Q, Koc H, Koc EC. Regulation of succinate dehydrogenase activity by SIRT3 in mammalian mitochondria. Biochemistry. 2010; 49:304–311.
Article29. Kosanam H, Thai K, Zhang Y, Advani A, Connelly KA, Diamandis EP, et al. Diabetes induces lysine acetylation of intermediary metabolism enzymes in the kidney. Diabetes. 2014; 63:2432–2439.
Article30. Palmieri F, Prezioso G, Quagliariello E, Klingenberg M. Kinetic study of the dicarboxylate carrier in rat liver mitochondria. Eur J Biochem. 1971; 22:66–74.
Article31. Ohana E, Shcheynikov N, Moe OW, Muallem S. SLC26A6 and NaDC-1 transporters interact to regulate oxalate and citrate homeostasis. J Am Soc Nephrol. 2013; 24:1617–1626.
Article32. Ariza AC, Deen PM, Robben JH. The succinate receptor as a novel therapeutic target for oxidative and metabolic stress-related conditions. Front Endocrinol (Lausanne). 2012; 3:22.
Article33. He W, Miao FJ, Lin DC, Schwandner RT, Wang Z, Gao J, et al. Citric acid cycle intermediates as ligands for orphan G-protein-coupled receptors. Nature. 2004; 429:188–193.
Article34. Mills E, O'Neill LA. Succinate: a metabolic signal in inflammation. Trends Cell Biol. 2014; 24:313–320.
Article35. Murphy MP, O'Neill LAJ. Krebs cycle reimagined: the emerging roles of succinate and itaconate as signal transducers. Cell. 2018; 174:780–784.
Article36. Correa PR, Kruglov EA, Thompson M, Leite MF, Dranoff JA, Nathanson MH. Succinate is a paracrine signal for liver damage. J Hepatol. 2007; 47:262–269.
Article37. Pluznick JL. Renal and cardiovascular sensory receptors and blood pressure regulation. Am J Physiol Renal Physiol. 2013; 305:F439–F444.
Article38. Robben JH, Fenton RA, Vargas SL, Schweer H, Peti-Peterdi J, Deen PM, et al. Localization of the succinate receptor in the distal nephron and its signaling in polarized MDCK cells. Kidney Int. 2009; 76:1258–1267.
Article39. Lei W, Ren W, Ohmoto M, Urban JF Jr, Matsumoto I, Margolskee RF, et al. Activation of intestinal tuft cell-expressed Sucnr1 triggers type 2 immunity in the mouse small intestine. Proc Natl Acad Sci U S A. 2018; 115:5552–5557.
Article40. Nadjsombati MS, McGinty JW, Lyons-Cohen MR, Jaffe JB, DiPeso L, Schneider C, et al. Detection of succinate by intestinal tuft cells triggers a type 2 innate immune circuit. Immunity. 2018; 49:33–41.
Article41. Wittenberger T, Schaller HC, Hellebrand S. An expressed sequence tag (EST) data mining strategy succeeding in the discovery of new G-protein coupled receptors. J Mol Biol. 2001; 307:799–813.42. Molnar T, Dobolyi A, Nyitrai G, Barabas P, Heja L, Emri Z, et al. Calcium signals in the nucleus accumbens: activation of astrocytes by ATP and succinate. BMC Neurosci. 2011; 12:96.43. Molnar T, Fekete EK, Kardos J, Simon-Trompler E, Palkovits M, Emri Z. Metabolic GHB precursor succinate binds to gamma-hydroxybutyrate receptors: characterization of human basal ganglia areas nucleus accumbens and globus pallidus. J Neurosci Res. 2006; 84:27–36.44. de Castro Fonseca M, Aguiar CJ, da Rocha Franco JA, Gingold RN, Leite MF. GPR91: expanding the frontiers of Krebs cycle intermediates. Cell Commun Signal. 2016; 14:3.
Article45. Deen PM, Robben JH. Succinate receptors in the kidney. J Am Soc Nephrol. 2011; 22:1416–1422.
Article46. Bratic A, Larsson NG. The role of mitochondria in aging. J Clin Invest. 2013; 123:951–957.
Article47. Ushio-Fukai M, Rehman J. Redox and metabolic regulation of stem/progenitor cells and their niche. Antioxid Redox Signal. 2014; 21:1587–1590.
Article48. Perales-Clemente E, Folmes CD, Terzic A. Metabolic regulation of redox status in stem cells. Antioxid Redox Signal. 2014; 21:1648–1659.
Article49. Levtchenko E, de Graaf-Hess A, Wilmer M, van den Heuvel L, Monnens L, Blom H. Altered status of glutathione and its metabolites in cystinotic cells. Nephrol Dial Transplant. 2005; 20:1828–1832.
Article50. Seidman MD, Bai U, Khan MJ, Quirk WS. Mitochondrial DNA deletions associated with aging and presbyacusis. Arch Otolaryngol Head Neck Surg. 1997; 123:1039–1045.
Article51. Orsucci D, Filosto M, Siciliano G, Mancuso M. Electron transfer mediators and other metabolites and cofactors in the treatment of mitochondrial dysfunction. Nutr Rev. 2009; 67:427–438.
Article52. Abdul-Ghani MA, Muller FL, Liu Y, Chavez AO, Balas B, Zuo P, et al. Deleterious action of FA metabolites on ATP synthesis: possible link between lipotoxicity, mitochondrial dysfunction, and insulin resistance. Am J Physiol Endocrinol Metab. 2008; 295:E678–E685.
Article53. Lange LG, Sobel BE. Mitochondrial dysfunction induced by fatty acid ethyl esters, myocardial metabolites of ethanol. J Clin Invest. 1983; 72:724–731.
Article54. Huang J, Lemire BD. Mutations in the C. elegans succinate dehydrogenase iron-sulfur subunit promote superoxide generation and premature aging. J Mol Biol. 2009; 387:559–569.
Article55. Walker DW, Hajek P, Muffat J, Knoepfle D, Cornelison S, Attardi G, et al. Hypersensitivity to oxygen and shortened lifespan in a Drosophila mitochondrial complex II mutant. Proc Natl Acad Sci U S A. 2006; 103:16382–16387.
Article56. Balietti M, Giorgetti B, Di Stefano G, Casoli T, Platano D, Solazzi M, et al. A ketogenic diet increases succinic dehydrogenase (SDH) activity and recovers age-related decrease in numeric density of SDH-positive mitochondria in cerebellar Purkinje cells of late-adult rats. Micron. 2010; 41:143–148.
Article57. Guo Y, Xie C, Li X, Yang J, Yu T, Zhang R, et al. Succinate and its G-protein-coupled receptor stimulates osteoclastogenesis. Nat Commun. 2017; 8:15621.
Article58. Littlewood-Evans A, Sarret S, Apfel V, Loesle P, Dawson J, Zhang J, et al. GPR91 senses extracellular succinate released from inflammatory macrophages and exacerbates rheumatoid arthritis. J Exp Med. 2016; 213:1655–1662.59. Shen J, Wang C, Ying J, Xu T, McAlinden A, O'Keefe RJ. Inhibition of 4-aminobutyrate aminotransferase protects against injury-induced osteoarthritis in mice. JCI Insight. 2019; 4:128568.
Article60. Al-Mashat HA, Kandru S, Liu R, Behl Y, Desta T, Graves DT. Diabetes enhances mRNA levels of proapoptotic genes and caspase activity, which contribute to impaired healing. Diabetes. 2006; 55:487–495.
Article61. Graves DT, Al-Mashat H, Liu R. Evidence that diabetes mellitus aggravates periodontal diseases and modifies the response to an oral pathogen in animal models. Compend Contin Educ Dent. 2004; 25:38–45.62. Pacios S, Kang J, Galicia J, Gluck K, Patel H, Ovaydi-Mandel A, et al. Diabetes aggravates periodontitis by limiting repair through enhanced inflammation. FASEB J. 2012; 26:1423–1430.
Article63. Wu YY, Xiao E, Graves DT. Diabetes mellitus related bone metabolism and periodontal disease. Int J Oral Sci. 2015; 7:63–72.
Article64. Lu RF, Feng XH, Xu L, Meng HX. Clinical and putative periodontal pathogens' features of different sites with probing depth reduction after non-surgical periodontal treatment of patients with aggressive periodontitis. Beijing Da Xue Xue Bao Yi Xue Ban. 2015; 47:13–18.65. Ohwaki K. High carboxylic acid level in the gingival crevicular fluid (GCF) of the patients with advanced periodontal disease. Nihon Shishubyo Gakkai Kaishi. 1988; 30:985–995.66. Su W, Shi J, Zhao Y, Yan F, Lei L, Li H. Porphyromonas gingivalis triggers inflammatory responses in periodontal ligament cells by succinate-succinate dehydrogenase-HIF-1α axis. Biochem Biophys Res Commun. 2020; 522:184–190.
Article67. Caimmi S, Caimmi D, Bousquet PJ, Demoly P. Succinate as opposed to glucocorticoid itself allergy. Allergy. 2008; 63:1641–1643.
Article68. Holz W, Ludwig A, Forst H. Anaphylactic shock following intravenous hydrocortisone succinate administration. Anaesthesist. 2002; 51:187–190.69. Calogiuri G, Foti C, Nettis E, Di Leo E, Macchia L, Vacca A. Should succinate esters be considered excipients in systemic corticosteroid allergy. J Allergy Clin Immunol Pract. 2019; 7:342–343.
Article70. Li PH, Wagner A, Thomas I, Watts TJ, Rutkowski R, Rutkowski K. Steroid allergy: clinical features and the importance of excipient testing in a diagnostic algorithm. J Allergy Clin Immunol Pract. 2018; 6:1655–1661.
Article71. Rubic-Schneider T, Carballido-Perrig N, Regairaz C, Raad L, Jost S, Rauld C, et al. GPR91 deficiency exacerbates allergic contact dermatitis while reducing arthritic disease in mice. Allergy. 2017; 72:444–452.
Article72. Chang C, Guo ZG, He B, Yao WZ. Metabolic alterations in the sera of Chinese patients with mild persistent asthma: a GC-MS-based metabolomics analysis. Acta Pharmacol Sin. 2015; 36:1356–1366.
Article73. Mathis D, Shoelson SE. Immunometabolism: an emerging frontier. Nat Rev Immunol. 2011; 11:81.
Article74. King A, Selak MA, Gottlieb E. Succinate dehydrogenase and fumarate hydratase: linking mitochondrial dysfunction and cancer. Oncogene. 2006; 25:4675–4682.
Article75. Jiang S, Yan W. Succinate in the cancer-immune cycle. Cancer Lett. 2017; 390:45–47.
Article76. Selak MA, Armour SM, MacKenzie ED, Boulahbel H, Watson DG, Mansfield KD, et al. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-alpha prolyl hydroxylase. Cancer Cell. 2005; 7:77–85.77. Masson N, Ratcliffe PJ. Hypoxia signaling pathways in cancer metabolism: the importance of co-selecting interconnected physiological pathways. Cancer Metab. 2014; 2:3.
Article78. Habano W, Sugai T, Nakamura S, Uesugi N, Higuchi T, Terashima M, et al. Reduced expression and loss of heterozygosity of the SDHD gene in colorectal and gastric cancer. Oncol Rep. 2003; 10:1375–1380.
Article79. Baysal BE. On the association of succinate dehydrogenase mutations with hereditary paraganglioma. Trends Endocrinol Metab. 2003; 14:453–459.
Article80. O'Neill LA, Pearce EJ. Immunometabolism governs dendritic cell and macrophage function. J Exp Med. 2016; 213:15–23.81. Rubic T, Lametschwandtner G, Jost S, Hinteregger S, Kund J, Carballido-Perrig N, et al. Triggering the succinate receptor GPR91 on dendritic cells enhances immunity. Nat Immunol. 2008; 9:1261–1269.
Article82. Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer. 2012; 12:265–277.
Article83. Wu JY, Huang TW, Hsieh YT, Wang YF, Yen CC, Lee GL, et al. Cancer-derived succinate promotes macrophage polarization and cancer metastasis via succinate receptor. Mol Cell. 2020; 77:213–227.
Article84. Ray I, Dasgupta A, De RK. Succinate aggravates NAFLD progression to liver cancer on the onset of obesity: an in silico model. J Bioinform Comput Biol. 2018; 16:1850008.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Succinate Induces Liver Damage and Hepatic Fibrosis in a Mouse Model
- Kisspeptin Regulation of Neuronal Activity throughout the Central Nervous System
- The use of intra-cellular signaling pathways in anesthesiology and pain medicine field
- Phloretin Ameliorates Succinate-Induced Liver Fibrosis by Regulating Hepatic Stellate Cells
- Wireless Energy and Data Transmission Using Inductive Coupling