Korean J Gastroenterol.
2020 Feb;75(2):103-107. 10.4166/kjg.2020.75.2.103.
Acute Acalculous Cholecystitis Associated with Sunitinib Treatment for Renal Cell Carcinoma
- Affiliations
-
- 1Department of Internal Medicine, Good Gang-An Hospital, Busan, Korea.
- 2Department of Internal Medicine, Samyook Busan Hospital, Busan, Korea.
- 3Division of Gastroenterology, Department of Internal Medicine, Dong-A University College of Medicine, Busan, Korea. mhnho@dau.ac.kr
- KMID: 2471150
- DOI: http://doi.org/10.4166/kjg.2020.75.2.103
Abstract
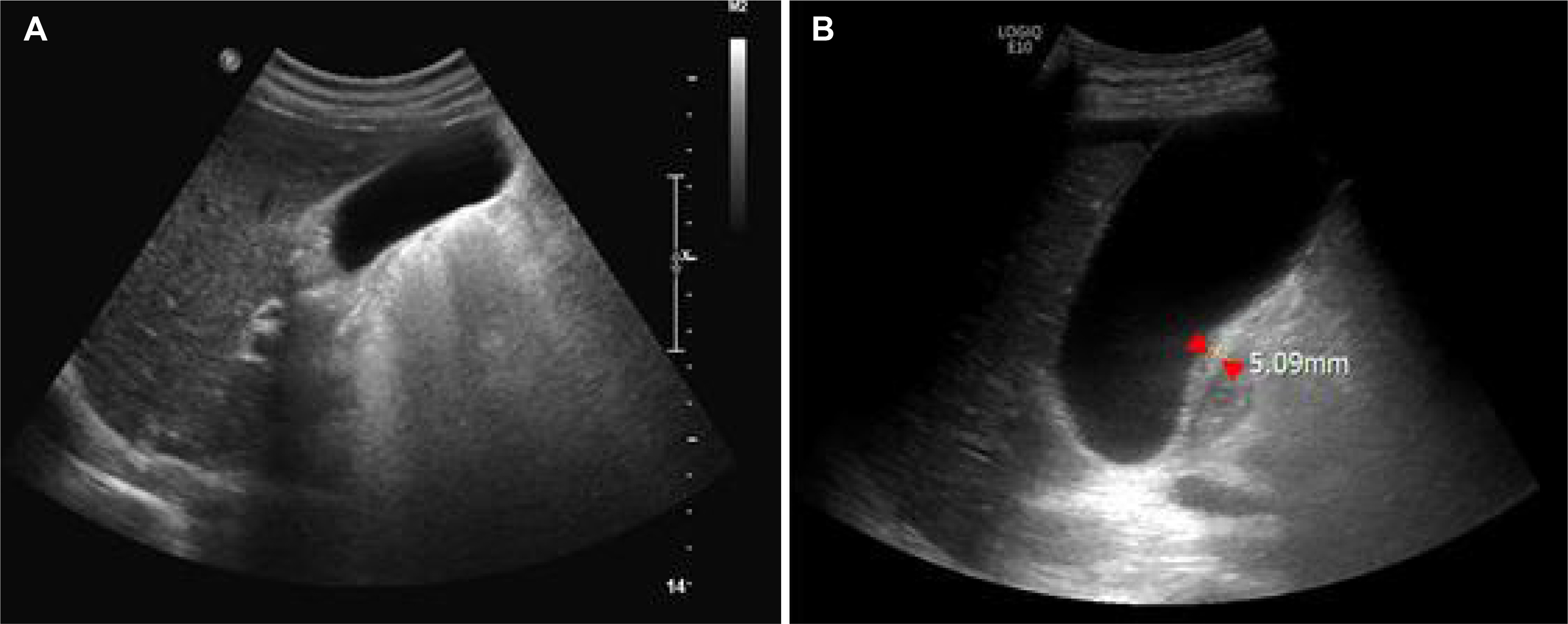
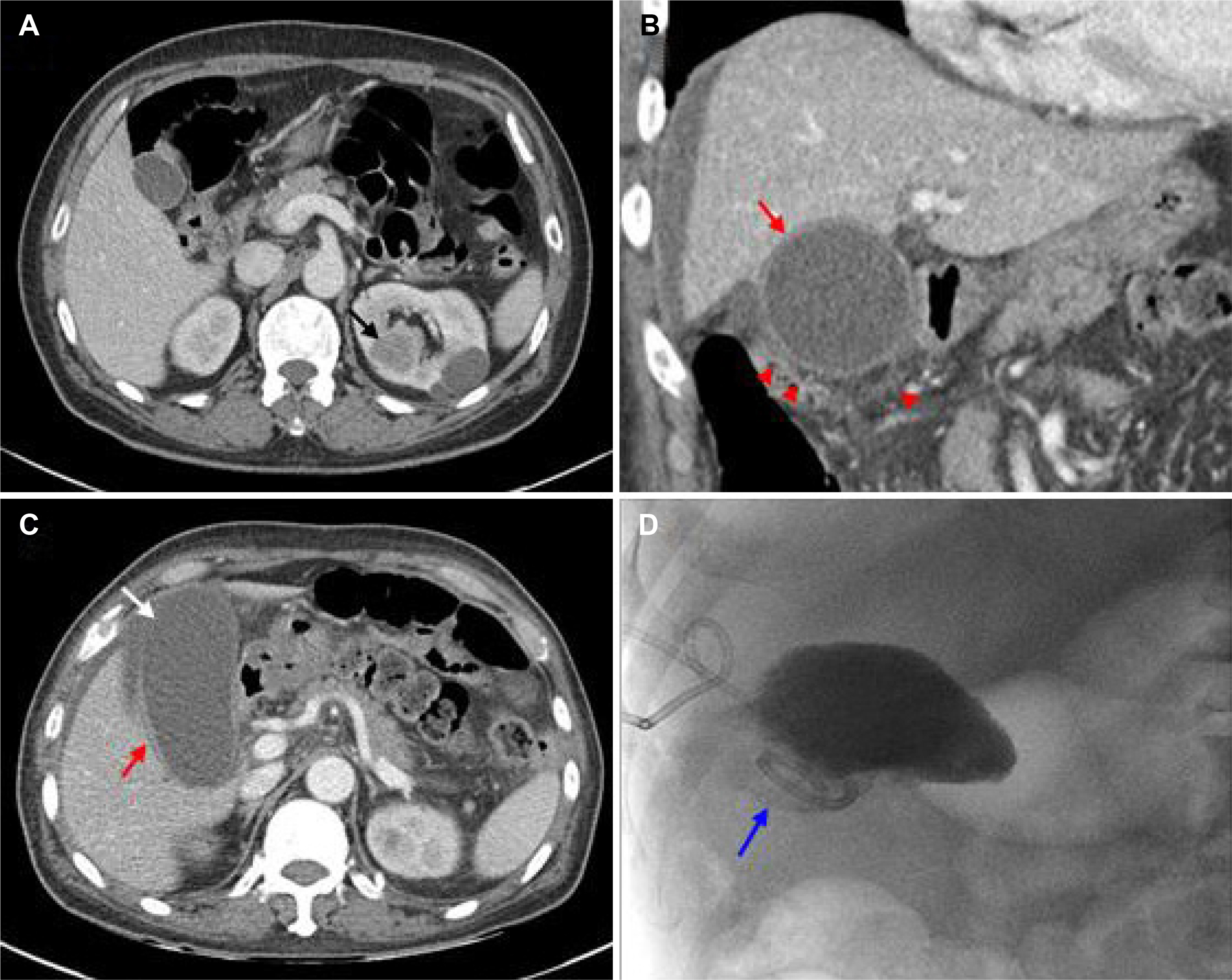
- A 64-year-old man was treated with sunitinib as a first-line therapy for metastatic renal cell carcinoma. He was given oral sunitinib in cycles of 50 mg once daily for 2 weeks followed by a week off. During the 5th week of treatment right upper quadrant pain developed, but this resolved spontaneously during the 6th week (off treatment). However, on the 8th week of treatment, he was admitted to hospital because the acute right upper quadrant pain recurred with nausea, vomiting, and fever. Acute acalculous cholecystitis was then diagnosed by ultrasonography and CT. In addition, his laboratory findings indicated disseminated intravascular coagulation. Accordingly, sunitinib therapy was discontinued and broad-spectrum antibiotics initiated. He subsequently recovered after emergent percutaneous cholecystostomy. His Naranjo Adverse Drug Reaction Probability Scale score was 7, indicaing a probable association of the event with sunitinib. Suspicion of sunitinib-related acute cholecystitis is required, because, although uncommon, it can be life-threatening.
MeSH Terms
Figure
Reference
-
References
1. Houk BE, Bello CL, Poland B, Rosen LS, Demetri GD, Motzer RJ. Relationship between exposure to sunitinib and efficacy and tolerability endpoints in patients with cancer: results of a pharma-cokinetic/pharmacodynamic metaanalysis. Cancer Chemother Pharmacol. 2010; 66:357–371.
Article2. Lee JL, Kim MK, Park I, et al. RandomizEd phase II trial of sunitinib four weeks on and two weeks off versus two weeks on and one week off in metastatic clear-cell type REnal cell carcinoma: RESTORE trial. Ann Oncol. 2015; 26:2300–2305.
Article3. de Lima Lopes G Jr, Rocha Lima CM. Emphysematous cholecystitis in a patient with gastrointestinal stromal tumor treated with sunitinib. Pharmacotherapy. 2007; 27:775–777.
Article4. Gomez-Abuin G, Karam AA, Mezzadri NA, Bas CA. Acalculous cholecystitis in a patient with metastatic renal cell carcinoma treated with sunitinib. Clin Genitourin Cancer. 2009; 7:62–63.
Article5. Nakano K, Suzuki K, Morita T. Life-threatening acute acalculous cholecystitis in a patient with renal cell carcinoma treated by sunitinib: a case report. J Med Case Rep. 2012; 6:69–72.
Article6. da Fonseca LG, Barroso-Sousa R, Sabbaga J, Hoff PM. Acute acalculous cholecystitis in a patient with metastatic renal cell carcinoma treated with sunitinib. Clin Pract. 2014; 4:635.7. Furubayashi N, Negishi T, Hirata Y, Taguchi K, Nakamura M. Acute acalculous cholecystitis in patients with clear cell renal cell carcinoma treated with sunitinib: report of two cases. J Clin Med Res. 2014; 6:302–304.
Article8. Tirumani SH, Krajewski KM, Shinagare AB, Jagannathan JP, Ramaiya NH. Gallbladder complications associated with molecular targeted therapies: clinical and imaging features. Clin Imaging. 2014; 38:50–55.
Article9. Laurila JJ, Ala-Kokko TI, Laurila PA, et al. Histopathology of acute acalculous cholecystitis in critically ill patients. Histopathology. 2005; 47:485–492.
Article10. Gotink KJ, Verheul HM. Anti-angiogenic tyrosine kinase inhibitors: what is their mechanism of action? Angiogenesis. 2010; 13:1–14.
Article11. Faivre S, Delbaldo C, Vera K, et al. Safety, pharmacokinetic, and antitumor activity of SU11248, a novel oral multitarget tyrosine kinase inhibitor, in patients with cancer. J Clin Oncol. 2006; 24:25–35.
Article12. Aihara Y, Yoshiji H, Yamazaki M, et al. A case of severe acalculous cholecystitis associated with sorafenib treatment for advanced hepatocellular carcinoma. World J Gastrointest Oncol. 2012; 4:115–118.
Article13. Kameda T, Nakano K, Yamazaki M, Koshimizu T, Morita T. Axitinib-induced pneumatosis intestinalis and acute acalculous cholecystitis in a patient with renal cell carcinoma. Urology. 2017; 101:e7–e8.
Article14. Rosen LS, Lipton L, Price TJ, et al. The effect of different dosing regimens of motesanib on the gallbladder: a randomized phase 1b study in patients with advanced solid tumors. BMC Cancer. 2013; 13:242.
Article15. Gaudio E, Barbaro B, Alvaro D, et al. Vascular endothelial growth factor stimulates rat cholangiocyte proliferation via an autocrine mechanism. Gastroenterology. 2006; 130:1270–1282.
Article16. Britten CD, Kabbinavar F, Hecht JR, et al. A phase I and pharmacokinetic study of sunitinib administered daily for 2 weeks, followed by a 1-week off period. Cancer Chemother Pharmacol. 2008; 61:515–524.17. Elice F, Rodeghiero F, Falanga A, Rickles FR. Thrombosis associated with angiogenesis inhibitors. Best Pract Res Clin Haematol. 2009; 22:115–128.
Article18. Di Paolo A, Bracarda S, Arrigoni E, Danesi R. Sunitinib in metastatic renal cell carcinoma: the pharmacological basis of the alternative 2/1 schedule. Front Pharmacol. 2017; 8:523.
Article19. Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981; 30:239–245.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Intolerance to Sunitinib Treatment in Hemodialysis Patients With Metastatic Renal Cell Carcinoma
- Hypoglycemic Coma in a Patient with Metastatic Renal Cell Carcinoma Treated with Sunitinib
- Acute acalculous cholecystitis
- Acalculous Hemorrhagic Cholecystitis with Chronic Intraluminal Hematoma: MRI Findings
- A Case of Acute Acalculous Cholecystitis in a Patient with Systemic Lupus Erythematosus