J Korean Assoc Oral Maxillofac Surg.
2020 Feb;46(1):3-11. 10.5125/jkaoms.2020.46.1.3.
Review of a novel disease entity, immunoglobulin G4-related disease
- Affiliations
-
- 1Section of Oral and Maxillofacial Oncology, Division of Maxillofacial Diagnostic and Surgical Sciences, Faculty of Dental Science, Kyushu University, Fukuoka, Japan. tmaehara@dent.kyushu-u.ac.jp
- KMID: 2471106
- DOI: http://doi.org/10.5125/jkaoms.2020.46.1.3
Abstract
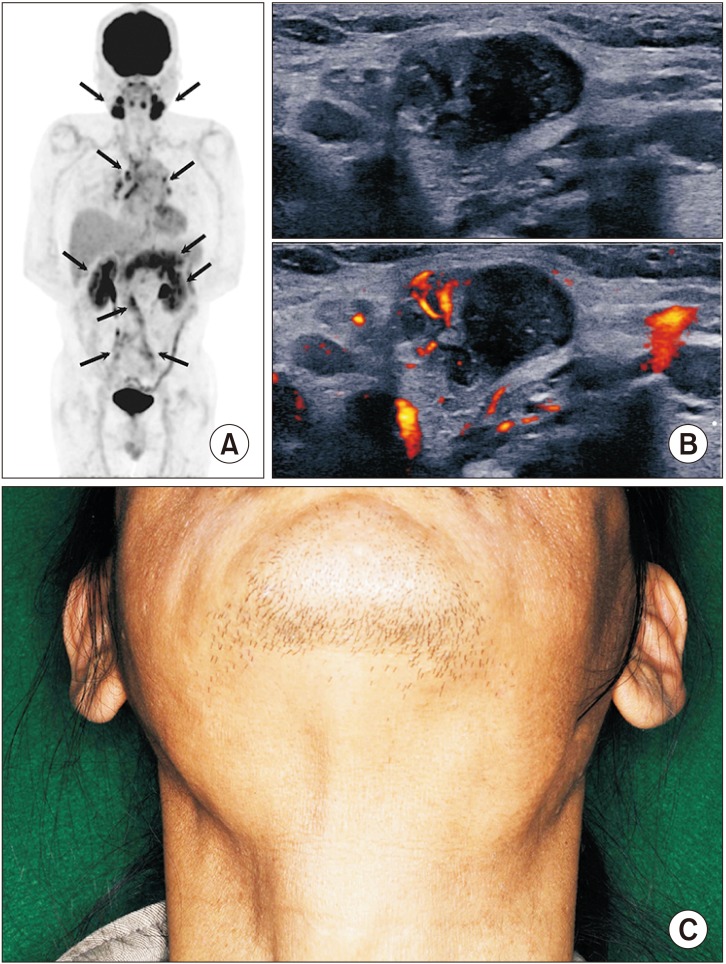
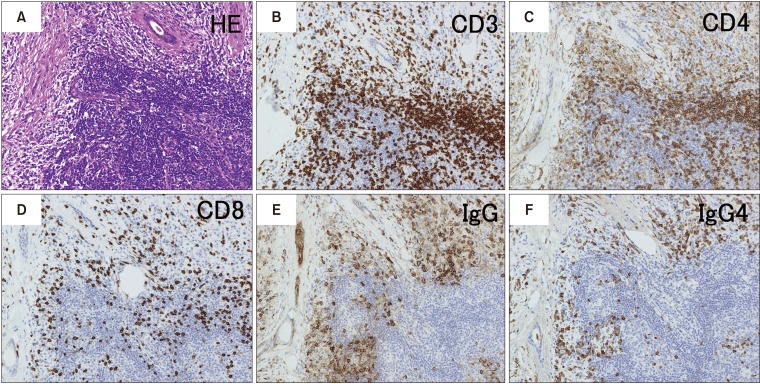
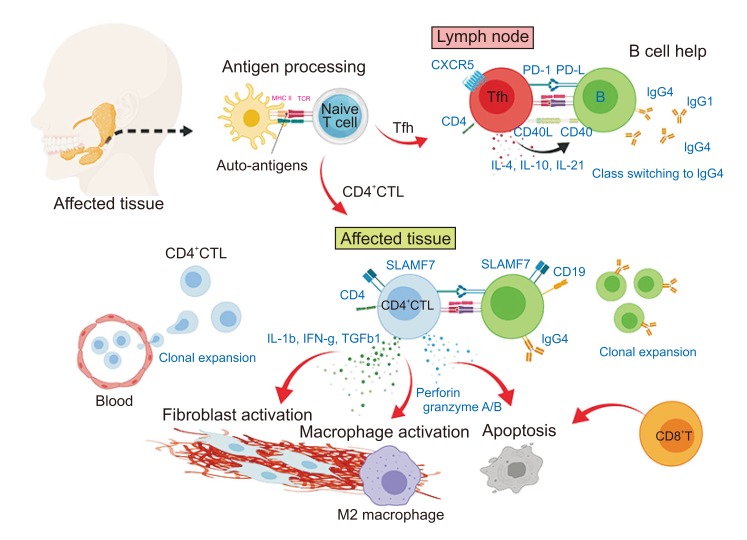
- Immunoglobulin G4 (IgG4)-related dacryoadenitis and sialoadenitis (IgG4-DS) are part of a multiorgan fibroinflammatory condition of unknown etiology termed IgG4-related disease (IgG4-RD), which has been recognized as a single diagnostic entity for less than 15 years. Histopathologic examination is critical for diagnosis of IgG4-RD. CD4+ T and B cells, including IgG4-expressing plasma cells, constitute the major inflammatory cell populations in IgG4-RD and are thought to cause organ damage and tissue fibrosis. Patients with IgG4-RD who have active, untreated disease exhibit significant increase of IgG4-secreting plasmablasts in the blood. Considerable insight into the immunologic mechanisms of IgG4-RD has been achieved in the last decade using novel molecular biology approaches, including next-generation and single-cell RNA sequencing. Exploring the interactions between CD4+ T cells and B lineage cells is critical for understanding the pathophysiology of IgG4-RD. Establishment of pathogenic T cell clones and identification of antigens specific to these clones constitutes the first steps in determining the pathogenesis of the disease. Herein, the clinical features and mechanistic insights regarding pathogenesis of IgG4-RD were reviewed.
Keyword
MeSH Terms
Figure
Reference
-
1. Kamisawa T, Zen Y, Pillai S, Stone JH. IgG4-related disease. Lancet. 2015; 385:1460–1471. PMID: 25481618.
Article2. Mahajan VS, Mattoo H, Deshpande V, Pillai SS, Stone JH. IgG4-related disease. Annu Rev Pathol. 2014; 9:315–347. PMID: 24111912.
Article3. Furukawa S, Moriyama M, Kawano S, Tanaka A, Maehara T, Hayashida JN, et al. Clinical relevance of Küttner tumour and IgG4-related dacryoadenitis and sialoadenitis. Oral Dis. 2015; 21:257–262. PMID: 24844187.4. Deshpande V, Zen Y, Chan JK, Yi EE, Sato Y, Yoshino T, et al. Consensus statement on the pathology of IgG4-related disease. Mod Pathol. 2012; 25:1181–1192. PMID: 22596100.
Article5. Khosroshahi A, Wallace ZS, Crowe JL, Akamizu T, Azumi A, Carruthers MN, et al. International consensus guidance statement on the management and treatment of IgG4-related disease. Arthritis Rheumatol. 2015; 67:1688–1699. PMID: 25809420.
Article6. Wallace ZS, Zhang Y, Perugino CA, Naden R, Choi HK, Stone JH. Clinical phenotypes of IgG4-related disease: an analysis of two international cross-sectional cohorts. Ann Rheum Dis. 2019; 78:406–412. PMID: 30612117.
Article7. Shimizu M, Okamura K, Kise Y, Takeshita Y, Furuhashi H, Weerawanich W, et al. Effectiveness of imaging modalities for screening IgG4-related dacryoadenitis and sialadenitis (Mikulicz's disease) and for differentiating it from Sjögren's syndrome (SS), with an emphasis on sonography. Arthritis Res Ther. 2015; 17:223. PMID: 26298875.
Article8. Sakamoto M, Moriyama M, Shimizu M, Chinju A, Mochizuki K, Munemura R, et al. The diagnostic utility of submandibular gland sonography and labial salivary gland biopsy in IgG4-related dacryoadenitis and sialadenitis: Its potential application to the diagnostic criteria. Mod Rheumatol. 2019; DOI: 10.1080/14397595.2019.1576271. [Epub ahead of print].
Article9. Maehara T, Pillai S, Stone JH, Nakamura S. Clinical features and mechanistic insights regarding IgG4-related dacryoadenitis and sialoadenitis: a review. Int J Oral Maxillofac Surg. 2019; 48:908–916. PMID: 30686634.
Article10. Umehara H, Okazaki K, Nakamura T, Satoh-Nakamura T, Nakajima A, Kawano M, et al. Current approach to the diagnosis of IgG4-related disease - combination of comprehensive diagnostic and organ-specific criteria. Mod Rheumatol. 2017; 27:381–391. PMID: 28165852.
Article11. Li W, Chen Y, Sun ZP, Cai ZG, Li TT, Zhang L, et al. Clinicopathological characteristics of immunoglobulin G4-related sialadenitis. Arthritis Res Ther. 2015; 17:186. PMID: 26194097.
Article12. Moriyama M, Furukawa S, Kawano S, Goto Y, Kiyoshima T, Tanaka A, et al. The diagnostic utility of biopsies from the submandibular and labial salivary glands in IgG4-related dacryoadenitis and sialoadenitis, so-called Mikulicz's disease. Int J Oral Maxillofac Surg. 2014; 43:1276–1281. PMID: 25062551.
Article13. Kawa S, Ota M, Yoshizawa K, Horiuchi A, Hamano H, Ochi Y, et al. HLA DRB10405-DQB10401 haplotype is associated with autoimmune pancreatitis in the Japanese population. Gastroenterology. 2002; 122:1264–1269. PMID: 11984513.
Article14. Terao C, Ota M, Iwasaki T, Shiokawa M, Kawaguchi S, Kuriyama K, et al. IgG4-related disease in the Japanese population: a genome-wide association study. Lancet Rheumatol. 2019; 1:PE14–PE22.
Article15. Strehl JD, Hartmann A, Agaimy A. Numerous IgG4-positive plasma cells are ubiquitous in diverse localised non-specific chronic inflammatory conditions and need to be distinguished from IgG4-related systemic disorders. J Clin Pathol. 2011; 64:237–243. PMID: 21233087.
Article16. Bruhns P, Iannascoli B, England P, Mancardi DA, Fernandez N, Jorieux S, et al. Specificity and affinity of human Fcgamma receptors and their polymorphic variants for human IgG subclasses. Blood. 2009; 113:3716–3725. PMID: 19018092.17. Carruthers MN, Khosroshahi A, Augustin T, Deshpande V, Stone JH. The diagnostic utility of serum IgG4 concentrations in IgG4-related disease. Ann Rheum Dis. 2015; 74:14–18. PMID: 24651618.
Article18. Maehara T. [IgG4-related disease -mechanistic insights from both clinical and immunologic understanding of this condition]. Nihon Rinsho Meneki Gakkai Kaishi. 2017; 40:206–212. Japanese. PMID: 28747608.
Article19. Mattoo H, Stone JH, Pillai S. Clonally expanded cytotoxic CD4+ T cells and the pathogenesis of IgG4-related disease. Autoimmunity. 2017; 50:19–24. PMID: 28166682.
Article20. Mattoo H, Mahajan VS, Maehara T, Deshpande V, Della-Torre E, Wallace ZS, et al. Clonal expansion of CD4(+) cytotoxic T lymphocytes in patients with IgG4-related disease. J Allergy Clin Immunol. 2016; 138:825–838. PMID: 26971690.
Article21. Mattoo H, Mahajan VS, Della-Torre E, Sekigami Y, Carruthers M, Wallace ZS, et al. De novo oligoclonal expansions of circulating plasmablasts in active and relapsing IgG4-related disease. J Allergy Clin Immunol. 2014; 134:679–687. PMID: 24815737.
Article22. Haldar D, Hirschfield GM. Deciphering the biology of IgG4-related disease: specific antigens and disease? Gut. 2018; 67:602–605. PMID: 29101259.
Article23. Carruthers MN, Topazian MD, Khosroshahi A, Witzig TE, Wallace ZS, Hart PA, et al. Rituximab for IgG4-related disease: a prospective, open-label trial. Ann Rheum Dis. 2015; 74:1171–1177. PMID: 25667206.
Article24. Della-Torre E, Feeney E, Deshpande V, Mattoo H, Mahajan V, Kulikova M, et al. B-cell depletion attenuates serological biomarkers of fibrosis and myofibroblast activation in IgG4-related disease. Ann Rheum Dis. 2015; 74:2236–2243. PMID: 25143523.
Article25. Tanaka A, Moriyama M, Nakashima H, Miyake K, Hayashida JN, Maehara T, et al. Th2 and regulatory immune reactions contribute to IgG4 production and the initiation of Mikulicz disease. Arthritis Rheum. 2012; 64:254–263. PMID: 21898360.
Article26. Maehara T, Mattoo H, Ohta M, Mahajan VS, Moriyama M, Yamauchi M, et al. Lesional CD4+ IFN-γ+ cytotoxic T lymphocytes in IgG4-related dacryoadenitis and sialoadenitis. Ann Rheum Dis. 2017; 76:377–385. PMID: 27358392.
Article27. Tian Y, Sette A, Weiskopf D. Cytotoxic CD4 T cells: differentiation, function, and application to dengue virus infection. Front Immunol. 2016; 7:531. PMID: 28003809.
Article28. Wallace ZS, Mattoo H, Carruthers M, Mahajan VS, Della Torre E, Lee H, et al. Plasmablasts as a biomarker for IgG4-related disease, independent of serum IgG4 concentrations. Ann Rheum Dis. 2015; 74:190–195. PMID: 24817416.
Article29. Della-Torre E, Bozzalla-Cassione E, Sciorati C, Ruggiero E, Lanzillotta M, Bonfiglio S, et al. A CD8α- subset of CD4+SLAMF7+ cytotoxic T cells is expanded in patients with IgG4-related disease and decreases following glucocorticoid treatment. Arthritis Rheumatol. 2018; 70:1133–1143. PMID: 29499100.
Article30. Pillai S, Mattoo H, Cariappa A. B cells and autoimmunity. Curr Opin Immunol. 2011; 23:721–731. PMID: 22119110.
Article31. Maehara T, Moriyama M, Nakamura S. Pathogenesis of IgG4-related disease: a critical review. Odontology. 2019; 107:127–132. PMID: 30019169.
Article32. Crotty S. Follicular helper CD4 T cells (TFH). Annu Rev Immunol. 2011; 29:621–663. PMID: 21314428.
Article33. Akiyama M, Suzuki K, Yamaoka K, Yasuoka H, Takeshita M, Kaneko Y, et al. Number of circulating follicular helper 2 T cells correlates with IgG4 and interleukin-4 levels and plasmablast numbers in IgG4-related disease. Arthritis Rheumatol. 2015; 67:2476–2481. PMID: 25989153.34. Akiyama M, Yasuoka H, Yamaoka K, Suzuki K, Kaneko Y, Kondo H, et al. Enhanced IgG4 production by follicular helper 2 T cells and the involvement of follicular helper 1 T cells in the pathogenesis of IgG4-related disease. Arthritis Res Ther. 2016; 18:167. PMID: 27411315.
Article35. Grados A, Ebbo M, Piperoglou C, Groh M, Regent A, Samson M, et al. T cell polarization toward TH2/TFH2 and TH17/TFH17 in patients with IgG4-related disease. Front Immunol. 2017; 8:235. PMID: 28348556.
Article36. Du H, Shi L, Chen P, Yang W, Xun Y, Yang C, et al. Prohibitin is involved in patients with IgG4 related disease. PLoS One. 2015; 10:e0125331. PMID: 25932630.
Article37. Hubers LM, Vos H, Schuurman AR, Erken R, Oude Elferink RP, Burgering B, et al. Annexin A11 is targeted by IgG4 and IgG1 autoantibodies in IgG4-related disease. Gut. 2018; 67:728–735. PMID: 28765476.
Article38. Shiokawa M, Kodama Y, Sekiguchi K, Kuwada T, Tomono T, Kuriyama K, et al. Laminin 511 is a target antigen in autoimmune pancreatitis. Sci Transl Med. 2018; 10:eaaq0997. PMID: 30089633.
Article39. Liu H, Perugino CA, Ghebremichael M, Wallace ZS, Montesi SB, Stone JH, et al. Disease severity is linked to an increase in autoantibody diversity in IgG4-related disease. Arthritis Rheumatol. 2019; DOI: 10.1002/art.41140. [Epub ahead of print].40. Tsuboi H, Nakai Y, Iizuka M, Asashima H, Hagiya C, Tsuzuki S, et al. DNA microarray analysis of labial salivary glands in IgG4-related disease: comparison with Sjögren's syndrome. Arthritis Rheumatol. 2014; 66:2892–2899. PMID: 24943710.
Article41. Tsuboi H, Matsuo N, Iizuka M, Tsuzuki S, Kondo Y, Tanaka A, et al. Analysis of IgG4 class switch-related molecules in IgG4-related disease. Arthritis Res Ther. 2012; 14:R171. PMID: 22824292.
Article42. Furukawa S, Moriyama M, Miyake K, Nakashima H, Tanaka A, Maehara T, et al. Interleukin-33 produced by M2 macrophages and other immune cells contributes to Th2 immune reaction of IgG4-related disease. Sci Rep. 2017; 7:42413. PMID: 28205524.
Article43. Watanabe T, Yamashita K, Fujikawa S, Sakurai T, Kudo M, Shiokawa M, et al. Involvement of activation of toll-like receptors and nucleotide-binding oligomerization domain-like receptors in enhanced IgG4 responses in autoimmune pancreatitis. Arthritis Rheum. 2012; 64:914–924. PMID: 21971969.
Article44. Watanabe T, Yamashita K, Sakurai T, Kudo M, Shiokawa M, Uza N, et al. Toll-like receptor activation in basophils contributes to the development of IgG4-related disease. J Gastroenterol. 2013; 48:247–253. PMID: 22744834.
Article45. Ishiguro N, Moriyama M, Furusho K, Furukawa S, Shibata T, Murakami Y, et al. Activated M2 macrophages contribute to the pathogenesis of IgG4-related disease via toll-like receptor 7/interleukin-33 signaling. Arthritis Rheumatol. 2020; 72:166–178. PMID: 31339007.
Article46. Kamisawa T, Shimosegawa T, Okazaki K, Nishino T, Watanabe H, Kanno A, et al. Standard steroid treatment for autoimmune pancreatitis. Gut. 2009; 58:1504–1507. PMID: 19398440.
Article47. Masaki Y, Matsui S, Saeki T, Tsuboi H, Hirata S, Izumi Y, et al. A multicenter phase II prospective clinical trial of glucocorticoid for patients with untreated IgG4-related disease. Mod Rheumatol. 2017; 27:849–854. PMID: 27846767.
Article48. Hong X, Zhang YY, Li W, Liu YY, Wang Z, Chen Y, et al. Treatment of immunoglobulin G4-related sialadenitis: outcomes of glucocorticoid therapy combined with steroid-sparing agents. Arthritis Res Ther. 2018; 20:12. PMID: 29382364.
Article49. Masamune A, Nishimori I, Kikuta K, Tsuji I, Mizuno N, Iiyama T, et al. Randomised controlled trial of long-term maintenance corticosteroid therapy in patients with autoimmune pancreatitis. Gut. 2017; 66:487–494. PMID: 27543430.
Article50. Shimosegawa T, Chari ST, Frulloni L, Kamisawa T, Kawa S, Mino-Kenudson M, et al. International consensus diagnostic criteria for autoimmune pancreatitis: guidelines of the International Association of Pancreatology. Pancreas. 2011; 40:352–358. PMID: 21412117.51. Moriyama M, Tanaka A, Maehara T, Ohyama Y, Shimizu M, Nakashima H, et al. Clinical characteristics of Mikulicz's disease as an IgG4-related disease. Clin Oral Investig. 2013; 17:1995–2002.
Article52. Maehara T, Mattoo H, Mahajan VS, Murphy SJ, Yuen GJ, Ishiguro N, et al. The expansion in lymphoid organs of IL-4+ BATF+ T follicular helper cells is linked to IgG4 class switching in vivo. Life Sci Alliance. 2018; 1:e201800050. PMID: 29984361.
Article53. Wallace ZS, Deshpande V, Stone JH. Ophthalmic manifestations of IgG4-related disease: single-center experience and literature review. Semin Arthritis Rheum. 2014; 43:806–817. PMID: 24513111.
Article54. Baer AN, Gourin CG, Westra WH, Cox DP, Greenspan JS, Daniels TE, et al. Rare diagnosis of IgG4-related systemic disease by lip biopsy in an international Sjögren syndrome registry. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013; 115:e34–e39.
Article55. Yamamoto M, Takahashi H, Sugai S, Imai K. Clinical and pathological characteristics of Mikulicz's disease (IgG4-related plasmacytic exocrinopathy). Autoimmun Rev. 2005; 4:195–200. PMID: 15893711.
Article56. Hu EK, Parrish C, Wrobel B, Deshpande V, Stone JH. Immunoglobulin G4-related disease presenting as an ethmoid and maxillary mass. Ann Allergy Asthma Immunol. 2013; 111:75–77. PMID: 23806471.
Article57. Dahlgren M, Khosroshahi A, Nielsen GP, Deshpande V, Stone JH. Riedel's thyroiditis and multifocal fibrosclerosis are part of the IgG4-related systemic disease spectrum. Arthritis Care Res (Hoboken). 2010; 62:1312–1318. PMID: 20506114.
Article58. Cheuk W, Chan JK. Lymphadenopathy of IgG4-related disease: an underdiagnosed and overdiagnosed entity. Semin Diagn Pathol. 2012; 29:226–234. PMID: 23068302.
Article59. Stone JH, Zen Y, Deshpande V. IgG4-related disease. N Engl J Med. 2012; 366:539–551. PMID: 22316447.
Article60. Khosroshahi A, Carruthers MN, Stone JH, Shinagare S, Sainani N, Hasserjian RP, et al. Rethinking Ormond's disease: "idiopathic" retroperitoneal fibrosis in the era of IgG4-related disease. Medicine (Baltimore). 2013; 92:82–91. PMID: 23429355.61. Inoue D, Zen Y, Abo H, Gabata T, Demachi H, Kobayashi T, et al. Immunoglobulin G4-related lung disease: CT findings with pathologic correlations. Radiology. 2009; 251:260–270. PMID: 19221056.
Article62. Saeki T, Nishi S, Imai N, Ito T, Yamazaki H, Kawano M, et al. Clinicopathological characteristics of patients with IgG4-related tubulointerstitial nephritis. Kidney Int. 2010; 78:1016–1023. PMID: 20720530.
Article63. Stone JH, Khosroshahi A, Deshpande V, Chan JK, Heathcote JG, Aalberse R, et al. Recommendations for the nomenclature of IgG4-related disease and its individual organ system manifestations. Arthritis Rheum. 2012; 64:3061–3067. PMID: 22736240.
Article64. Zen Y, Harada K, Sasaki M, Sato Y, Tsuneyama K, Haratake J, et al. IgG4-related sclerosing cholangitis with and without hepatic inflammatory pseudotumor, and sclerosing pancreatitis-associated sclerosing cholangitis: do they belong to a spectrum of sclerosing pancreatitis? Am J Surg Pathol. 2004; 28:1193–1203. PMID: 15316319.65. Mattoo H, Della-Torre E, Mahajan VS, Stone JH, Pillai S. Circulating Th2 memory cells in IgG4-related disease are restricted to a defined subset of subjects with atopy. Allergy. 2014; 69:399–402. PMID: 24382311.
Article66. Maehara T, Moriyama M, Nakashima H, Miyake K, Hayashida JN, Tanaka A, et al. Interleukin-21 contributes to germinal centre formation and immunoglobulin G4 production in IgG4-related dacryoadenitis and sialoadenitis, so-called Mikulicz's disease. Ann Rheum Dis. 2012; 71:2011–2019. PMID: 22753386.
Article67. Akiyama M, Suzuki K, Yasuoka H, Kaneko Y, Yamaoka K, Takeuchi T. Follicular helper T cells in the pathogenesis of IgG4-related disease. Rheumatology (Oxford). 2018; 57:236–245. PMID: 28460058.
Article68. Kamekura R, Takano K, Yamamoto M, Kawata K, Shigehara K, Jitsukawa S, et al. Cutting edge: a critical role of lesional T follicular helper cells in the pathogenesis of IgG4-related disease. J Immunol. 2017; 199:2624–2629. PMID: 28916523.
Article69. Kamekura R, Yamamoto M, Takano K, Yabe H, Ito F, Ikegami I, et al. Circulating PD-1+CXCR5-CD4+ T cells underlying the immunological mechanisms of IgG4-related disease. Rheumatol Adv Pract. 2018; 2:rky043. PMID: 31431980.
Article70. Perugino CA, AlSalem SB, Mattoo H, Della-Torre E, Mahajan V, Ganesh G, et al. Identification of galectin-3 as an autoantigen in patients with IgG4-related disease. J Allergy Clin Immunol. 2019; 143:736–745.e6.. PMID: 29852256.
Article71. Shiokawa M, Kodama Y, Kuriyama K, Yoshimura K, Tomono T, Morita T, et al. Pathogenicity of IgG in patients with IgG4-related disease. Gut. 2016; 65:1322–1332. PMID: 26964842.
Article72. Furukawa S, Moriyama M, Tanaka A, Maehara T, Tsuboi H, Iizuka M, et al. Preferential M2 macrophages contribute to fibrosis in IgG4-related dacryoadenitis and sialoadenitis, so-called Mikulicz's disease. Clin Immunol. 2015; 156:9–18. PMID: 25450336.
Article73. Ohta M, Moriyama M, Maehara T, Gion Y, Furukawa S, Tanaka A, et al. DNA microarray analysis of submandibular glands in IgG4-related disease indicates a role for MARCO and other innate Immune-related proteins. Medicine (Baltimore). 2016; 95:e2853. PMID: 26886650.
Article74. Perugino CA, Stone JH. Treatment of IgG4-related disease: current and future approaches. Z Rheumatol. 2016; 75:681–686. PMID: 27431746.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Immunoglobulin G4-related sclerosing cholangitis
- Immunoglobulin G4 Related Ophthalmic Disease Presenting as Diplopia and Headache
- A Case of Recurrent Ischemic Stroke Associated with Immunoglobulin G4-Related Disease
- Hepatic Immunoglobulin G4-related Inflammatory Pseudotumor Mimicking Hepatocellular Carcinoma
- Seven Cases of Immunoglobulin G4-Related Disease in Submandibular Glands