J Breast Cancer.
2019 Dec;22(4):653-660. 10.4048/jbc.2019.22.e45.
Salivary Duct Cancer Metastasis Mimicking Primary Breast Cancer: A Case Report and Review
- Affiliations
-
- 1Department of Surgery, King Faisal University, Al Ahsa, Saudi Arabia.
- 2Department of Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. doorkeeper1@gmail.com
- 3Department of Pathology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 4Department of Radiology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- KMID: 2470902
- DOI: http://doi.org/10.4048/jbc.2019.22.e45
Abstract
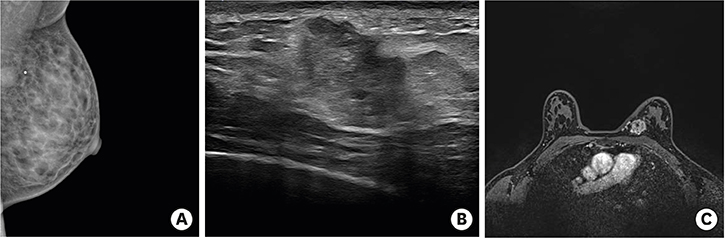
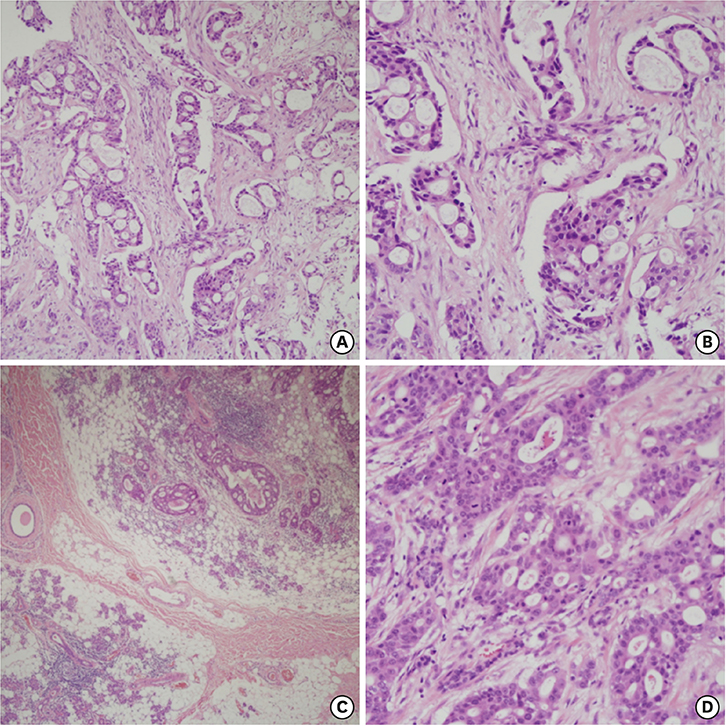
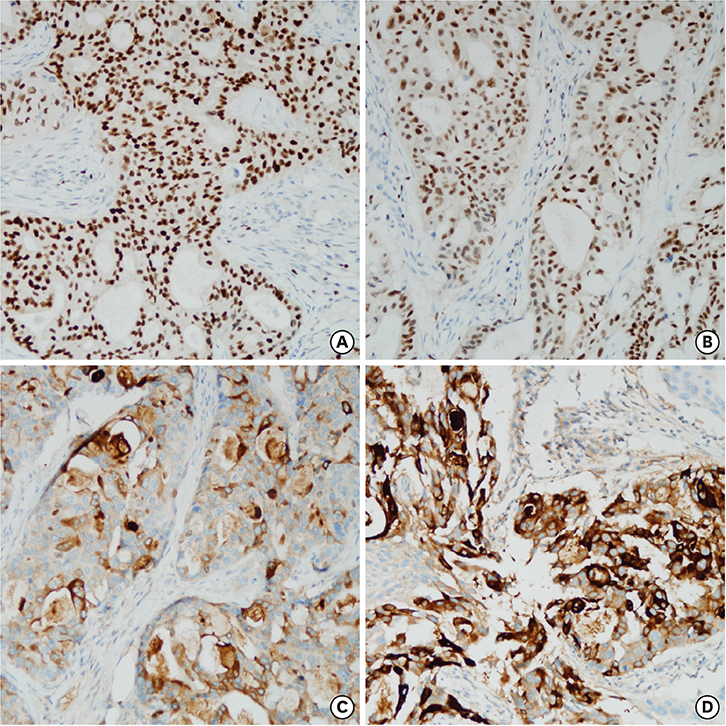
- Secondary breast malignancy is a rare occurrence, accounting for less than 2% of all breast malignancies. Salivary duct carcinoma (SDC) metastasizing to the breast has not been reported previously. This report presents the case of a woman who was initially diagnosed with and treated for parotid carcinoma. Two years later, during a follow-up visit, a breast lump was discovered, which was diagnosed as primary breast cancer and managed surgically. After surgery, hematoxylin and eosin and immunohistochemical staining revealed that the breast tumor had the same features as her primary SDC. Here, we present a confusing case of SDC metastasis to the breast that mimicked triple-negative breast cancer.
MeSH Terms
Figure
Reference
-
1. DeLair DF, Corben AD, Catalano JP, Vallejo CE, Brogi E, Tan LK. Non-mammary metastases to the breast and axilla: a study of 85 cases. Mod Pathol. 2013; 26:343–349.
Article2. Miettinen M, McCue PA, Sarlomo-Rikala M, Rys J, Czapiewski P, Wazny K, et al. GATA3: a multispecific but potentially useful marker in surgical pathology: a systematic analysis of 2500 epithelial and nonepithelial tumors. Am J Surg Pathol. 2014; 38:13–22.3. Satoh F, Umemura S, Osamura RY. Immunohistochemical analysis of GCDFP-15 and GCDFP-24 in mammary and non-mammary tissue. Breast Cancer. 2000; 7:49–55.
Article4. Surov A, Fiedler E, Holzhausen HJ, Ruschke K, Schmoll HJ, Spielmann RP. Metastases to the breast from non-mammary malignancies: primary tumors, prevalence, clinical signs, and radiological features. Acad Radiol. 2011; 18:565–574.5. Bohman LG, Bassett LW, Gold RH, Voet R. Breast metastases from extramammary malignancies. Radiology. 1982; 144:309–312.
Article6. Sippo DA, Kulkarni K, Carlo PD, Lee B, Eisner D, Cimino-Mathews A, et al. Metastatic disease to the breast from extramammary malignancies: a multimodality pictorial review. Curr Probl Diagn Radiol. 2016; 45:225–232.
Article7. Lee SK, Kim WW, Kim SH, Hur SM, Kim S, Choi JH, et al. Characteristics of metastasis in the breast from extramammary malignancies. J Surg Oncol. 2010; 101:137–140.
Article8. Akçay MN. Metastatic disease in the breast. Breast. 2002; 11:526–528.
Article9. Moncada R, Cooper RA, Garces M, Badrinath K. Calcified metastases from malignant ovarian neoplasm. Review of the literature. Radiology. 1974; 113:31–35.
Article10. Lee AH. The histological diagnosis of metastases to the breast from extramammary malignancies. J Clin Pathol. 2007; 60:1333–1341.
Article11. Fan CY, Wang J, Barnes EL. Expression of androgen receptor and prostatic specific markers in salivary duct carcinoma: an immunohistochemical analysis of 13 cases and review of the literature. Am J Surg Pathol. 2000; 24:579–586.
Article12. Kapadia SB, Barnes L. Expression of androgen receptor, gross cystic disease fluid protein, and CD44 in salivary duct carcinoma. Mod Pathol. 1998; 11:1033–1038.13. Huang HC, Hang JF, Wu MH, Chou TY, Chiu CH. Lung adenocarcinoma with ipsilateral breast metastasis: a simple coincidence? J Thorac Oncol. 2013; 8:974–979.
Article14. Mun SH, Ko EY, Han BK, Shin JH, Kim SJ, Cho EY. Breast metastases from extramammary malignancies: typical and atypical ultrasound features. Korean J Radiol. 2014; 15:20–28.
Article15. Cardenosa G. Breast Imaging. Philadelphia (PA): Lippincott Williams & Wilkins;2004. p. 270–276.