J Breast Cancer.
2020 Feb;23(1):80-92. 10.4048/jbc.2020.23.e8.
Prognostic Value of Skeletal Muscle Depletion Measured on Computed Tomography for Overall Survival in Patients with Non-Metastatic Breast Cancer
- Affiliations
-
- 1Department of Radiology, Ajou University School of Medicine, Suwon, Korea. h219435@gmail.com
- 2Department of Biomedical Informatics, Ajou University School of Medicine, Suwon, Korea.
- 3Department of Nuclear Medicine and Molecular Imaging, Ajou University School of Medicine, Suwon, Korea.
- 4Department of Surgery, Ajou University School of Medicine, Suwon, Korea.
- 5Department of Radiology, University of Ulsan, College of Medicine, Asan Medical Center, Suwon, Korea.
- KMID: 2470884
- DOI: http://doi.org/10.4048/jbc.2020.23.e8
Abstract
- PURPOSE
The purpose of this study was to evaluate the prognostic value of skeletal muscle depletion measured on computed tomography (CT) in patients with non-metastatic invasive breast cancer.
METHODS
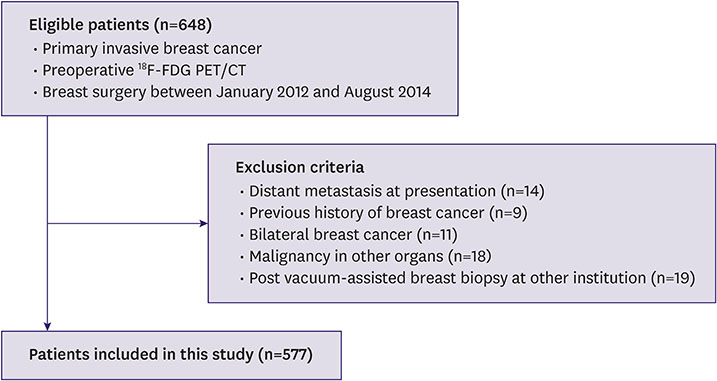
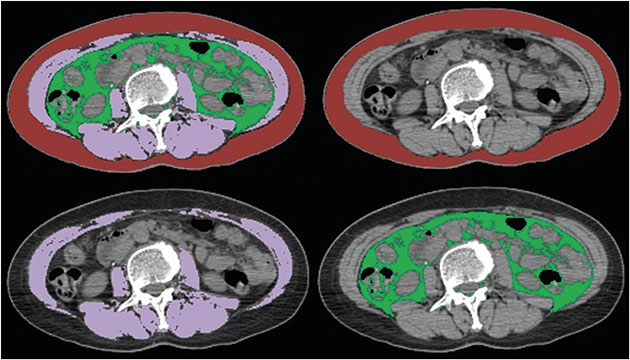
This retrospective study included 577 consecutive women (mean age ± standard deviation: 48.9 ± 10.2 years with breast cancer who underwent a preoperative positron-emission tomography (PET)/CT scan and curative surgery between January 2012 and August 2014. The total abdominal muscle area (TAMA), subcutaneous fat area (SFA), and visceral fat area (VFA) were measured on CT images at the L3 vertebral level. Univariate and multivariate Cox proportional-hazard regression analyses were performed to evaluate whether there was an association between sarcopenia and overall survival (OS) outcome.
RESULTS
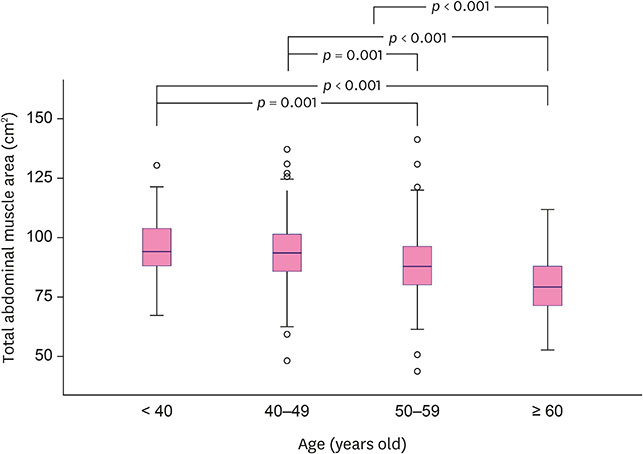
Of the 577 women, 49 (8.5%) died after a mean of 46 months. The best TAMA threshold for predicting OS was 83.7 cm². The multivariate Cox proportional-hazard analysis revealed that sarcopenia (TAMA ≤ 83.70 cm²) was a strong prognostic biomarker (hazard ratio [HR], 1.951; 95% confidence interval [CI], 1.061-3.586), along with large tumor size, axillary lymph node metastasis, high nuclear grade, estrogen receptor status, and adjuvant radiation therapy. In the subgroup analysis of patients aged ≥ 50 years, TAMA (≤ 77.14 cm²) was a significant independent factor (HR, 2.856; 95% CI, 1.218-6.695).
CONCLUSION
Skeletal muscle depletion measured on CT was associated with worse OS outcome in patients with non-metastatic breast cancer.
Keyword
MeSH Terms
Figure
Reference
-
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018; 68:7–30.
Article2. Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Peto R, Davies C, Godwin J, Gray R, Pan HC, et al. Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet. 2012; 379:432–444.
Article3. Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Davies C, Godwin J, Gray R, Clarke M, Cutter D, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011; 378:771–784.
Article4. O'Sullivan CC, Bradbury I, Campbell C, Spielmann M, Perez EA, Joensuu H, et al. Efficacy of Adjuvant trastuzumab for patients with human epidermal growth factor receptor 2-positive early breast cancer and tumors ≤ 2 cm: a meta-analysis of the randomized trastuzumab trials. J Clin Oncol. 2015; 33:2600–2608.5. DeSantis C, Ma J, Bryan L, Jemal A. Breast cancer statistics, 2013. CA Cancer J Clin. 2014; 64:52–62.
Article6. Adami HO, Malker B, Holmberg L, Persson I, Stone B. The relation between survival and age at diagnosis in breast cancer. N Engl J Med. 1986; 315:559–563.
Article7. Foulkes WD, Grainge MJ, Rakha EA, Green AR, Ellis IO. Tumor size is an unreliable predictor of prognosis in basal-like breast cancers and does not correlate closely with lymph node status. Breast Cancer Res Treat. 2009; 117:199–204.
Article8. Harvey JM, Clark GM, Osborne CK, Allred DC. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol. 1999; 17:1474–1481.
Article9. Chia S, Norris B, Speers C, Cheang M, Gilks B, Gown AM, et al. Human epidermal growth factor receptor 2 overexpression as a prognostic factor in a large tissue microarray series of node-negative breast cancers. J Clin Oncol. 2008; 26:5697–5704.
Article10. Ho-Huynh A, Tran A, Bray G, Abbot S, Elston T, Gunnarsson R, et al. Factors influencing breast cancer outcomes in Australia: a systematic review. Eur J Cancer Care (Engl). 2019; 28:e13038.
Article11. Lee K, Shin Y, Huh J, Sung YS, Lee IS, Yoon KH, et al. Recent issues on body composition imaging for sarcopenia evaluation. Korean J Radiol. 2019; 20:205–217.
Article12. Villaseñor A, Ballard-Barbash R, Baumgartner K, Baumgartner R, Bernstein L, McTiernan A, et al. Prevalence and prognostic effect of sarcopenia in breast cancer survivors: the HEAL study. J Cancer Surviv. 2012; 6:398–406.
Article13. Caan BJ, Cespedes Feliciano EM, Prado CM, Alexeeff S, Kroenke CH, Bradshaw P, et al. Association of muscle and adiposity measured by computed tomography with survival in patients with nonmetastatic breast cancer. JAMA Oncol. 2018; 4:798–804.
Article14. Song EJ, Lee CW, Jung SY, Kim BN, Lee KS, Lee S, et al. Prognostic impact of skeletal muscle volume derived from cross-sectional computed tomography images in breast cancer. Breast Cancer Res Treat. 2018; 172:425–436.
Article15. Weinberg MS, Shachar SS, Muss HB, Deal AM, Popuri K, Yu H, et al. Beyond sarcopenia: characterization and integration of skeletal muscle quantity and radiodensity in a curable breast cancer population. Breast J. 2018; 24:278–284.
Article16. Shachar SS, Deal AM, Weinberg M, Nyrop KA, Williams GR, Nishijima TF, et al. Skeletal muscle measures as predictors of toxicity, hospitalization, and survival in patients with metastatic breast cancer receiving taxane-based chemotherapy. Clin Cancer Res. 2017; 23:658–665.
Article17. Aleixo GF, Williams GR, Nyrop KA, Muss HB, Shachar SS. Muscle composition and outcomes in patients with breast cancer: meta-analysis and systematic review. Breast Cancer Res Treat. 2019; 177:569–579.
Article18. Yang M, Shen Y, Tan L, Li W. Prognostic value of sarcopenia in lung cancer: a systematic review and meta-analysis. Chest. 2019; 156:101–111.19. Kamarajah SK, Bundred J, Tan BH. Body composition assessment and sarcopenia in patients with gastric cancer: a systematic review and meta-analysis. Gastric Cancer. 2019; 22:10–22.
Article20. Mintziras I, Miligkos M, Wächter S, Manoharan J, Maurer E, Bartsch DK. Sarcopenia and sarcopenic obesity are significantly associated with poorer overall survival in patients with pancreatic cancer: systematic review and meta-analysis. Int J Surg. 2018; 59:19–26.
Article21. Nakamura N, Hara T, Shibata Y, Matsumoto T, Nakamura H, Ninomiya S, et al. Sarcopenia is an independent prognostic factor in male patients with diffuse large B-cell lymphoma. Ann Hematol. 2015; 94:2043–2053.
Article22. Moons KG, Altman DG, Reitsma JB, Ioannidis JP, Macaskill P, Steyerberg EW, et al. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med. 2015; 162:W1–73.
Article23. Youden WJ. Index for rating diagnostic tests. Cancer. 1950; 3:32–35.
Article24. Gonzalez MC, Pastore CA, Orlandi SP, Heymsfield SB. Obesity paradox in cancer: new insights provided by body composition. Am J Clin Nutr. 2014; 99:999–1005.
Article25. Lennon H, Sperrin M, Badrick E, Renehan AG. The obesity paradox in cancer: a review. Curr Oncol Rep. 2016; 18:56.
Article26. Kaji H. Effects of myokines on bone. Bonekey Rep. 2016; 5:826.
Article27. Beaudart C, Zaaria M, Pasleau F, Reginster JY, Bruyère O. Health outcomes of sarcopenia: a systematic review and meta-analysis. PLoS One. 2017; 12:e0169548.
Article28. Bazzocchi A, Diano D, Ponti F, Salizzoni E, Albisinni U, Marchesini G, et al. A 360-degree overview of body composition in healthy people: relationships among anthropometry, ultrasonography, and dual-energy x-ray absorptiometry. Nutrition. 2014; 30:696–701.
Article29. Prado CM, Baracos VE, McCargar LJ, Reiman T, Mourtzakis M, Tonkin K, et al. Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clin Cancer Res. 2009; 15:2920–2926.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Breast Cancer Metastatic to Gluteus Maximus: A Case Report
- Skeletal Muscle Metastases from Breast Cancer: Two Case Reports
- The Impact of the Ratio of Positive Nodes to Removed Nodes on Recurrence and Overall Survival in Node Positive Breast Cancer Patients
- The Metastatic Rate of Internal Mammary Lymph Nodes When Metastasis of Internal Mammary Lymph Node Is Suspected on PET/CT
- Redefining In-Breast Tumor Recurrence: Unveiling Metastatic Dynamics and Shifting the Focus to Overall Survival in Breast Cancer Surgery Assessment