J Breast Cancer.
2013 Jun;16(2):202-207. 10.4048/jbc.2013.16.2.202.
The Metastatic Rate of Internal Mammary Lymph Nodes When Metastasis of Internal Mammary Lymph Node Is Suspected on PET/CT
- Affiliations
-
- 1Department of Surgery, Yeungnam University College of Medicine, Daegu, Korea. cjegs@yu.ac.kr
- KMID: 2286402
- DOI: http://doi.org/10.4048/jbc.2013.16.2.202
Abstract
- PURPOSE
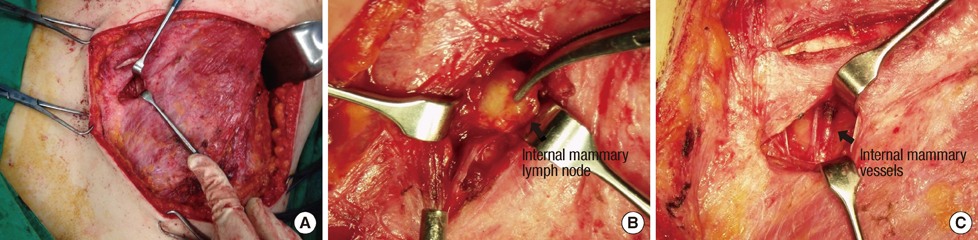
Metastatic status of internal mammary lymph node (IMLN) has a clinical importance in assessing the stage and prognosis of breast cancer. But, when metastasis of IMLN is suspected; the management is controversial. We retrospectively reviewed 36 breast cancer patients who underwent IMLN biopsy, and investigated the pathologic status of IMLN which suspected metastasis with positron emission tomography and computed tomography (PET/CT).
METHODS
From January 2007 to December 2012, 36 patients underwent IMLN biopsy for suspected IMLN metastasis on PET/CT, when diagnosed with primary or recurrent breast cancer. Clinicopathologic features of these patients and metastatic status of IMLNs were investigated.
RESULTS
A total of 36 patients were included in this study. Twenty-four patients diagnosed with primary breast cancer and 12 patients diagnosed with recurrent breast cancer underwent IMLN biopsy. The mean number of IMLNs was 2.72+/-2.05, and the total metastatic rate of IMLNs was 72.2% (26 out of 36). IMLN metastasis was confirmed on pathologic examination in 19 patients (79.2%, 19 out of 24) with primary breast cancer and in 7 patients (58.3%, 7 out of 12) with recurrent breast cancer. The mean standardized uptake values of metastatic and nonmetastatic IMLNs in primary breast cancer were 3.50+/-2.51 and 3.72+/-3.55, respectively and those of metastatic and nonmetastatic IMLN in recurrent breast cancer were 3.92+/-2.67 and 4.12+/-3.57, respectively. In both groups, there was no statistically significant difference between the SUVs of metastatic and nonmetastatic IMLNs (p=0.291 and p=0.951, respectively).
CONCLUSION
Due to the recent advances in diagnostic and surgical skills, IMLN biopsy can be performed safely without any complications without performing radical mastectomy. If IMLN metastasis is suspected on PET/CT, IMLN biopsy is useful to assess the exact stage and to determine the treatment for breast cancer. Further follow-up studies are needed to assess the locoregional recurrence and to compare the improvement in overall survival and disease-free survival.
Keyword
MeSH Terms
Figure
Reference
-
1. Veronesi U, Marubini E, Mariani L, Valagussa P, Zucali R. The dissection of internal mammary nodes does not improve the survival of breast cancer patients: 30-year results of a randomised trial. Eur J Cancer. 1999; 35:1320–1325.
Article2. Veronesi U, Cascinelli N, Greco M, Bufalino R, Morabito A, Galluzzo D, et al. Prognosis of breast cancer patients after mastectomy and dissection of internal mammary nodes. Ann Surg. 1985; 202:702–707.
Article3. Livingston SF, Arlen M. The extended extrapleural radical mastectomy: its role in the treatment of carcinoma of the breast. Ann Surg. 1974; 179:260–265.4. Donegan WL. The influence of untreated internal mammary metastases upon the course of mammary cancer. Cancer. 1977; 39:533–538.
Article5. Caceres E. Incidence of metastasis in the internal mammary chain in operable carcinoma of the breast and 5 year results. Acta Unio Int Contra Cancrum. 1963; 19:1566–1569.6. Urban JA, Marjani MA. Significance of internal mammary lymph node metastases in breast cancer. Am J Roentgenol Radium Ther Nucl Med. 1971; 111:130–136.
Article7. Lacour J, Le M, Caceres E, Koszarowski T, Veronesi U, Hill C. Radical mastectomy versus radical mastectomy plus internal mammary dissection. Ten year results of an international cooperative trial in breast cancer. Cancer. 1983; 51:1941–1943.
Article8. Veronesi U, Valagussa P. Inefficacy of internal mammary nodes dissection in breast cancer surgery. Cancer. 1981; 47:170–175.
Article9. Estourgie SH, Nieweg OE, Olmos RA, Rutgers EJ, Kroon BB. Lymphatic drainage patterns from the breast. Ann Surg. 2004; 239:232–237.
Article10. Hultborn KA, Larsson LG, Ragnhult I. The lymph drainage from the breast to the axillary and parasternal lymph nodes, studied with the aid of colloidal Au198. Acta Radiol. 1955; 43:52–64.
Article11. Huang O, Wang L, Shen K, Lin H, Hu Z, Liu G, et al. Breast cancer subpopulation with high risk of internal mammary lymph nodes metastasis: analysis of 2,269 Chinese breast cancer patients treated with extended radical mastectomy. Breast Cancer Res Treat. 2008; 107:379–387.
Article12. Handley RS, Thackray AC. Invasion of internal mammary lymph nodes in carcinoma of the breast. Br Med J. 1954; 1:61–63.
Article13. Lacour J, Lê MG, Hill C, Kramar A, Contesso G, Sarrazin D. Is it useful to remove internal mammary nodes in operable breast cancer? Eur J Surg Oncol. 1987; 13:309–314.14. Meier P, Ferguson DJ, Karrison T. A controlled trial of extended radical versus radical mastectomy. Ten-year results. Cancer. 1989; 63:188–195.
Article15. Morimoto T, Monden Y, Takashima S, Itoh S, Kimura T, Yamamoto H, et al. Five-year results of a randomized clinical trial comparing modified radical mastectomy and extended radical mastectomy for stage II breast cancer. Surg Today. 1994; 24:210–214.
Article16. Bellon JR, Livingston RB, Eubank WB, Gralow JR, Ellis GK, Dunnwald LK, et al. Evaluation of the internal mammary lymph nodes by FDG-PET in locally advanced breast cancer (LABC). Am J Clin Oncol. 2004; 27:407–410.
Article17. Eubank WB, Mankoff DA, Takasugi J, Vesselle H, Eary JF, Shanley TJ, et al. 18Fluorodeoxyglucose positron emission tomography to detect mediastinal or internal mammary metastases in breast cancer. J Clin Oncol. 2001; 19:3516–3523.
Article18. Jones A, Bernstein V, Davis N, Bryce C, Wilson D, Mankoff D. Pilot feasibility study to assess the utility of PET scanning in the pre-operative evaluation of internal mammary nodes in breast cancer patients presenting with medial hemisphere tumors. Clin Positron Imaging. 1999; 2:331.
Article19. Halverson KJ, Taylor ME, Perez CA, Garcia DM, Myerson R, Philpott G, et al. Regional nodal management and patterns of failure following conservative surgery and radiation therapy for stage I and II breast cancer. Int J Radiat Oncol Biol Phys. 1993; 26:593–599.
Article20. Katz A, Strom EA, Buchholz TA, Thames HD, Smith CD, Jhingran A, et al. Locoregional recurrence patterns after mastectomy and doxorubicin-based chemotherapy: implications for postoperative irradiation. J Clin Oncol. 2000; 18:2817–2827.
Article21. Clarke M, Collins R, Darby S, Davies C, Elphinstone P, Evans E, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005; 366:2087–2106.
Article22. Chen RC, Lin NU, Golshan M, Harris JR, Bellon JR. Internal mammary nodes in breast cancer: diagnosis and implications for patient management: a systematic review. J Clin Oncol. 2008; 26:4981–4989.
Article23. Fowble B, Hanlon A, Freedman G, Nicolaou N, Hoffman J, Sigurdson E, et al. Internal mammary node irradiation neither decreases distant metastases nor improves survival in stage I and II breast cancer. Int J Radiat Oncol Biol Phys. 2000; 47:883–894.
Article24. Lê MG, Arriagada R, de Vathaire F, Dewar J, Fontaine F, Lacour J, et al. Can internal mammary chain treatment decrease the risk of death for patients with medial breast cancers and positive axillary lymph nodes? Cancer. 1990; 66:2313–2318.
Article25. Obedian E, Haffty BG. Internal mammary nodal irradiation in conservatively-managed breast cancer patients: is there a benefit? Int J Radiat Oncol Biol Phys. 1999; 44:997–1003.
Article26. Arriagada R, Lê MG, Mouriesse H, Fontaine F, Dewar J, Rochard F, et al. Long-term effect of internal mammary chain treatment. Results of a multivariate analysis of 1195 patients with operable breast cancer and positive axillary nodes. Radiother Oncol. 1988; 11:213–222.
Article27. Giordano SH, Kuo YF, Freeman JL, Buchholz TA, Hortobagyi GN, Goodwin JS. Risk of cardiac death after adjuvant radiotherapy for breast cancer. J Natl Cancer Inst. 2005; 97:419–424.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Supraclavicular Lymph Node Metastasis from Various Malignancies: Assessment with 18F-Fluorodeoxyglucose Positron Emission Tomography/CT, Contrast-Enhanced CT and Ultrasound
- Contralateral Internal Mammary Lymphadenopathy Mimicking Metastasis in a Patient with a History of Breast Cancer and Prior Interstitial Mammoplasty by Paraffin Injection: MRI, PET-CT, and Pathological Findings
- Two Cases of Transoral Resection of Retropharyngeal Lymph Node Metastasis from Papillary Thyroid Carcinoma Diagnosed by PET-CT Follow-Up after Lateral Neck Dissection
- Reliability of 18F-Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography in the Nodal Staging of Colorectal Cancer Patients
- The Lymphatic Drainage Pattern of Internal Mammary Sentinel Lymph Node Identified by Small Particle Radiotracer ((99m)Tc-Dextran 40) in Breast