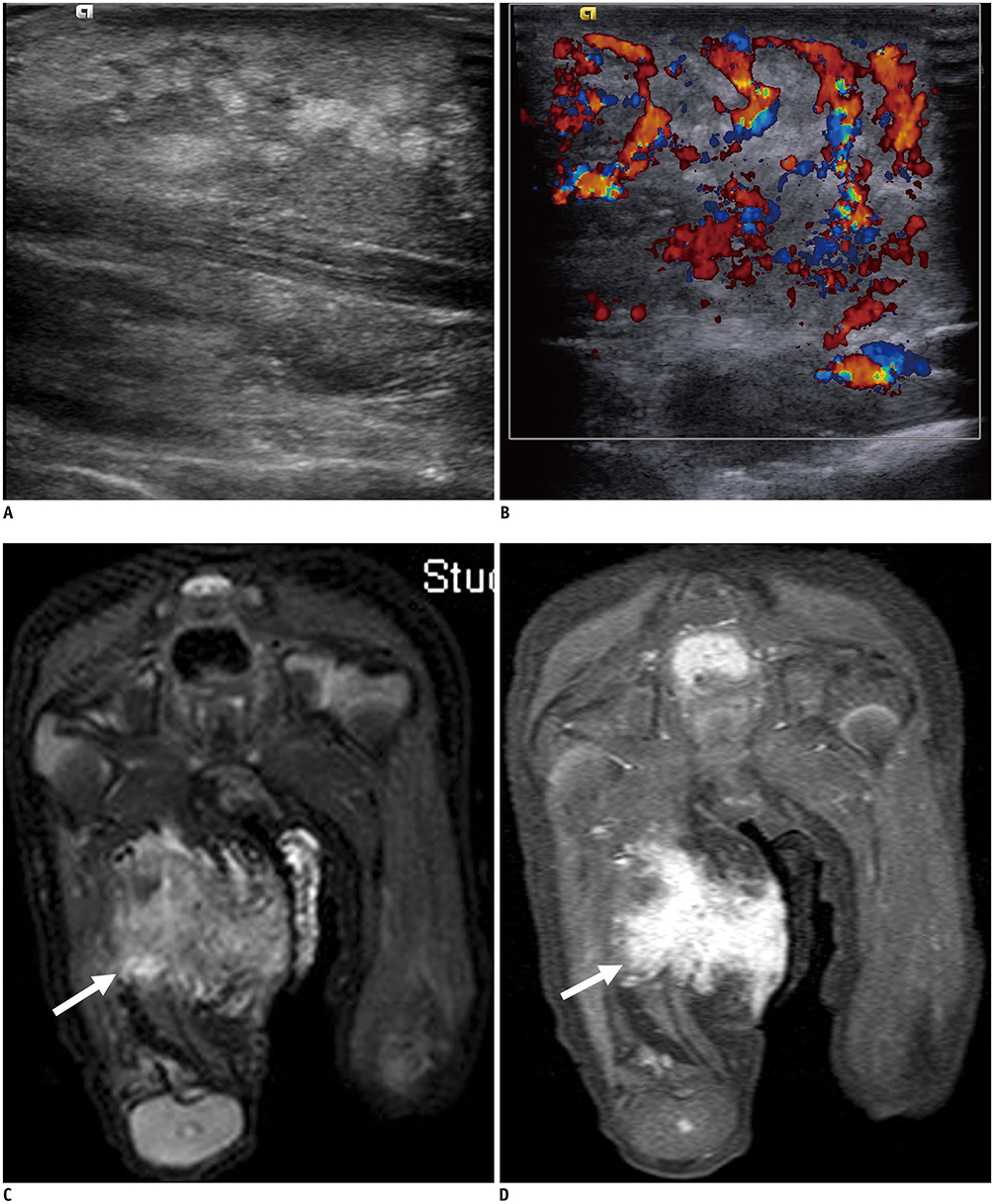
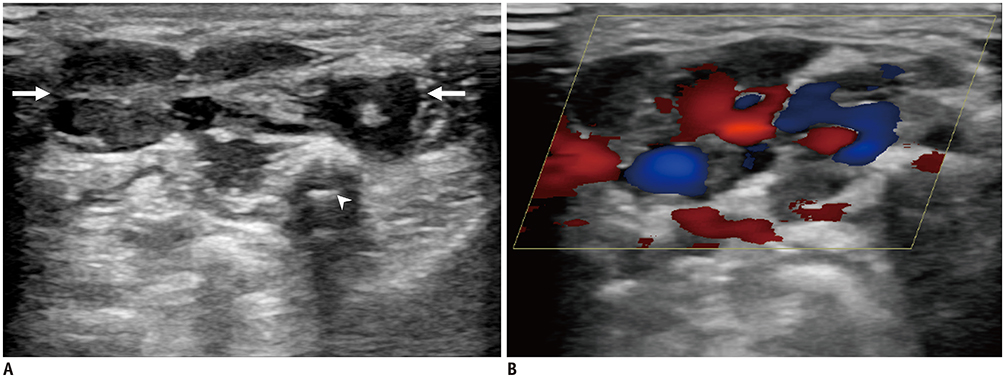
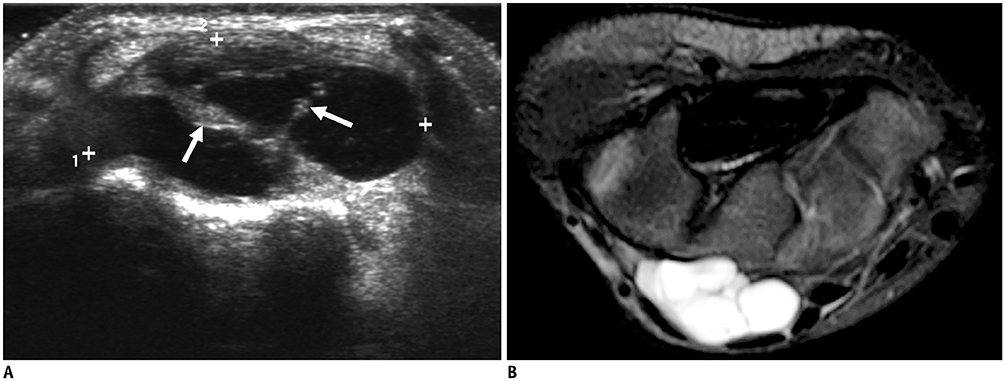
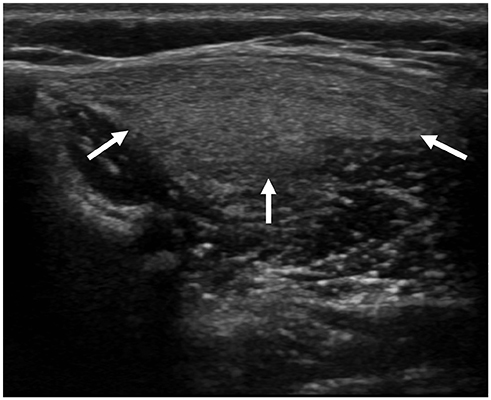
Korean J Radiol.
2020 Mar;21(3):341-355. 10.3348/kjr.2019.0343.
Ultrasonography of Pediatric Superficial Soft Tissue Tumors and Tumor-Like Lesions
- Affiliations
-
- 1Department of Radiology, Sungkyunkwan University School of Medicine, Samsung Medical Center, Seoul, Korea. sy1131.yoo@samsung.com
- 2Department of Radiology, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 3Department of Radiology, Korea University Medical Center, Seoul, Korea.
- KMID: 2470758
- DOI: http://doi.org/10.3348/kjr.2019.0343
Abstract
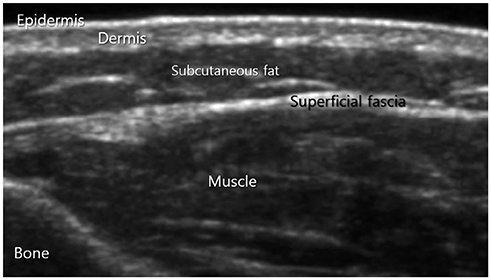
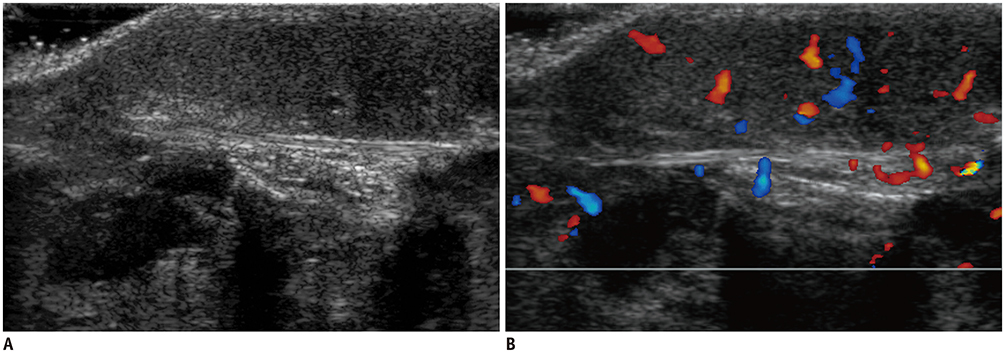
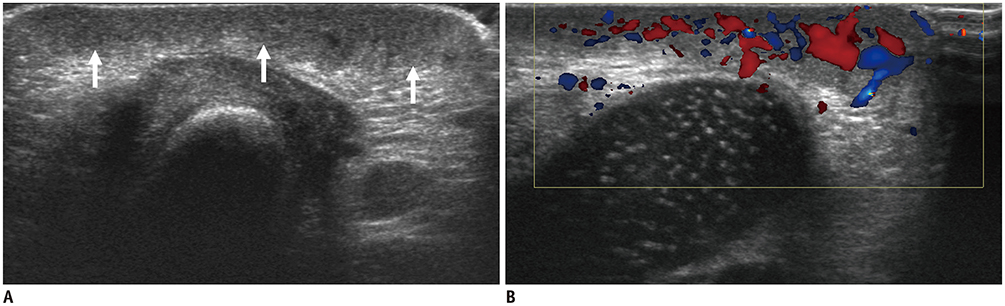
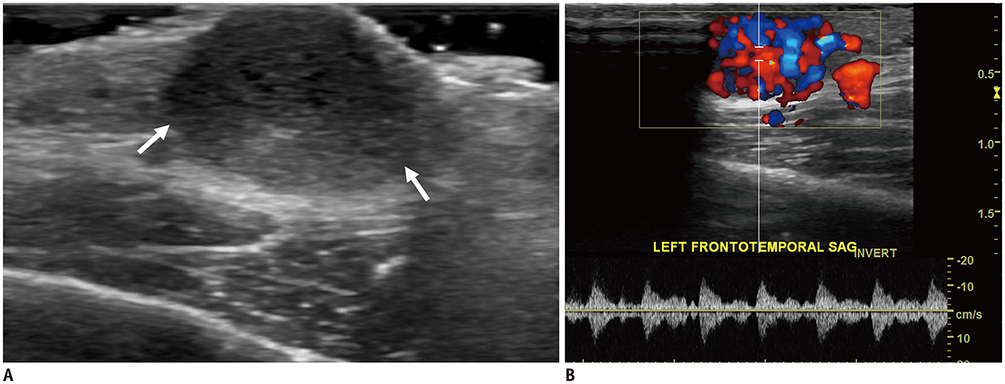
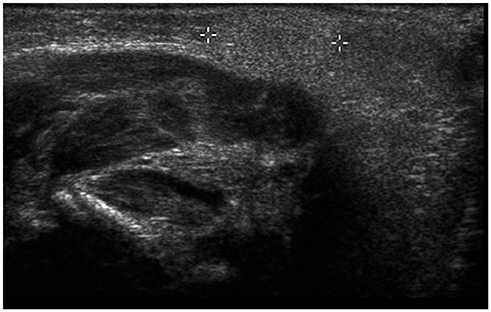
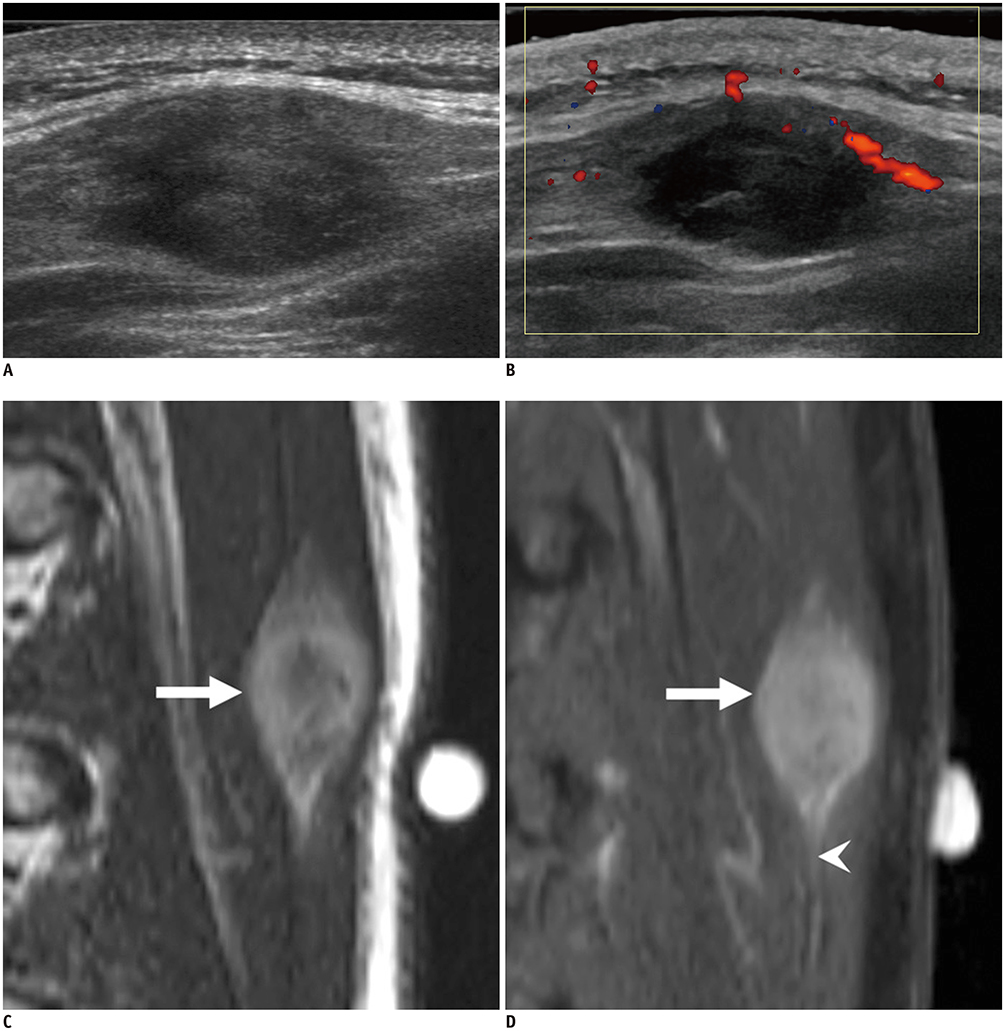
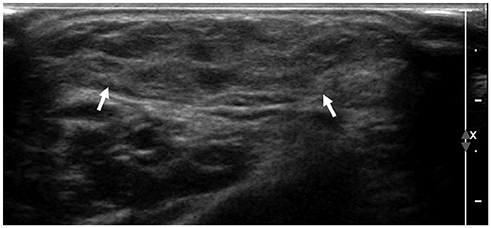
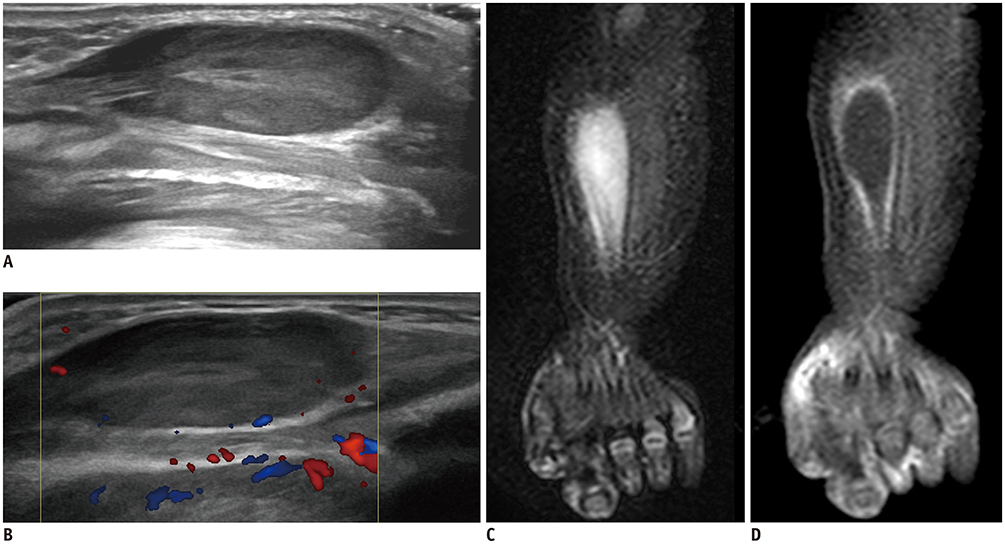
- Ultrasonography (US) is usually the first imaging examination performed to evaluate palpable or visible superficial soft tissue lesions that are common in children. Although clinical assessments, such as age at presentation, clinical course, and overlying skin discoloration, are important for the differentiation of pediatric soft tissue lesions, US allows a specific diagnosis of some typical benign lesions and helps in guiding further investigation since it provides detailed information about the lesion location, characterization including solid versus cystic, vascularity, and compressibility. Therefore, sufficient knowledge of the normal anatomy, proper ultrasonographic techniques, and the imaging findings of common and uncommon soft tissue lesions in children are crucial for accurate assessment and management of patients. In this article, we review the techniques and imaging findings focusing on the ultrasonographic features of a variety of superficial soft tissue lesions detected in children.
Keyword
Figure
Reference
-
1. Beaman FD, Kransdorf MJ, Andrews TR, Murphey MD, Arcara LK, Keeling JH. Superficial soft-tissue masses: analysis, diagnosis, and differential considerations. Radiographics. 2007; 27:509–523.
Article2. Wu JS, Hochman MG. Soft-tissue tumors and tumorlike lesions: a systematic imaging approach. Radiology. 2009; 253:297–316.
Article3. Navarro OM. Soft tissue masses in children. Radiol Clin North Am. 2011; 49:1235–1259. vi–vii.
Article4. Shah SH, Callahan MJ. Ultrasound evaluation of superficial lumps and bumps of the extremities in children: a 5-year retrospective review. Pediatr Radiol. 2013; 43 Suppl 1:S23–S40.
Article5. DiDomenico P, Middleton W. Sonographic evaluation of palpable superficial masses. Radiol Clin North Am. 2014; 52:1295–1305.
Article6. Carra BJ, Bui-Mansfield LT, O'Brien SD, Chen DC. Sonography of musculoskeletal soft-tissue masses: techniques, pearls, and pitfalls. AJR Am J Roentgenol. 2014; 202:1281–1290.
Article7. Campbell R. Ultrasound of soft tissue masses. In : Allan PL, Baxter GM, Weston MJ, editors. Clinical ultrasound. 2:3rd ed. London: Churchill Livingstone: Elsevier;2011. p. 1109–1125.8. Siegel MJ. Pediatric sonography. 5th ed. Philadelphia, PA: Wolters Kluwer;2019.9. ISSVA classification for vascular anomalies. ISSVA;2014. updated May 2018. Accessed September 22, 2019. Available at: http://www.issva.org/UserFiles/file/ISSVA-Classification-2018.pdf.10. Esposito F, Ferrara D, Di Serafino M, Diplomatico M, Vezzali N, Giugliano AM, et al. Classification and ultrasound findings of vascular anomalies in pediatric age: the essential. J Ultrasound. 2019; 22:13–25.
Article11. Enjolras O, Wassef M, Chapot R. Color atlas of vascular tumors and vascular malformations. 1st ed. Cambridge: Cambridge University Press;2007.12. Johnson CM, Navarro OM. Clinical and sonographic features of pediatric soft-tissue vascular anomalies part 1: classification, sonographic approach and vascular tumors. Pediatr Radiol. 2017; 47:1184–1195.
Article13. Olivieri B, White CL, Restrepo R, McKeon B, Karakas SP, Lee EY. Low-flow vascular malformation pitfalls: from clinical examination to practical imaging evaluation—Part 2, venous malformation mimickers. AJR Am J Roentgenol. 2016; 206:952–962.
Article14. Ryu YJ, Choi YH, Cheon JE, Kim WS, Kim IO, Park JE, et al. Imaging findings of kaposiform hemangioendothelioma in children. Eur J Radiol. 2017; 86:198–205.
Article15. White CL, Olivieri B, Restrepo R, McKeon B, Karakas SP, Lee EY. Low-flow vascular malformation pitfalls: from clinical examination to practical imaging evaluation—Part 1, lymphatic malformation mimickers. AJR Am J Roentgenol. 2016; 206:940–951.
Article16. Fishman SJ, Mulliken JB. Hemangiomas and vascular malformations of infancy and childhood. Pediatr Clin North Am. 1993; 40:1177–1200.
Article17. Johnson CM, Navarro OM. Clinical and sonographic features of pediatric soft-tissue vascular anomalies part 2: vascular malformations. Pediatr Radiol. 2017; 47:1196–1208.
Article18. Legiehn GM, Heran MK. Venous malformations: classification, development, diagnosis, and interventional radiologic management. Radiol Clin North Am. 2008; 46:545–597. vi
Article19. Flors L, Leiva-Salinas C, Maged IM, Norton PT, Matsumoto AH, Angle JF, et al. MR imaging of soft-tissue vascular malformations: diagnosis, classification, and therapy follow-up. Radiographics. 2011; 31:1321–1340. discussion 1340-1341.
Article20. Wassef M, Blei F, Adams D, Alomari A, Baselga E, Berenstein A, et al. ISSVA Board and Scientific Committee. Vascular anomalies classification: recommendations from the International Society for the Study of Vascular Anomalies. Pediatrics. 2015; 136:e203–e214.
Article21. Sheybani EF, Eutsler EP, Navarro OM. Fat-containing soft-tissue masses in children. Pediatr Radiol. 2016; 46:1760–1773.
Article22. Navarro OM, Laffan EE, Ngan BY. Pediatric soft-tissue tumors and pseudo-tumors: MR imaging features with pathologic correlation: part 1. Imaging approach, pseudotumors, vascular lesions, and adipocytic tumors. Radiographics. 2009; 29:887–906.23. Susam-Sen H, Yalcin B, Kutluk T, Cahit Tanyel F, Haliloglu M, Orhan D, et al. Lipoblastoma in children: review of 12 cases. Pediatr Int. 2017; 59:545–550.24. Han JW, Kim H, Youn JK, Oh C, Jung SE, Park KW, et al. Analysis of clinical features of lipoblastoma in children. Pediatr Hematol Oncol. 2017; 34:212–220.
Article25. Gupta P, Potti TA, Wuertzer SD, Lenchik L, Pacholke DA. Spectrum of fat-containing soft-tissue masses at MR imaging: the common, the uncommon, the characteristic, and the sometimes confusing. Radiographics. 2016; 36:753–766.
Article26. Szpecht D, Bagnosz-Magnuszewska A, Szymankiewicz M, Gadzinowski J. Subcutaneous fat necrosis in neonates after therapeutic hypothermia - report of two cases. Postepy Dermatol Alergol. 2016; 33:152–154.
Article27. Vasireddy S, Long SD, Sacheti B, Mayforth RD. MRI and US findings of subcutaneous fat necrosis of the newborn. Pediatr Radiol. 2009; 39:73–76.
Article28. Lee KJ, Jin W, Kim GY, Rhee SJ, Park SY, Park JS, et al. Sonographic features of superficial-type nodular fasciitis in the musculoskeletal system. J Ultrasound Med. 2015; 34:1465–1471.
Article29. Khuu A, Yablon CM, Jacobson JA, Inyang A, Lucas DR, Biermann JS. Nodular fasciitis: characteristic imaging features on sonography and magnetic resonance imaging. J Ultrasound Med. 2014; 33:565–573.30. Naidu A, Lerman MA. Clinical pathologic conference case 3: nodular fasciitis. Head Neck Pathol. 2011; 5:276–280.
Article31. Lee S, Choi YH, Cheon JE, Kim MJ, Lee MJ, Koh MJ. Ultrasonographic features of fibrous hamartoma of infancy. Skeletal Radiol. 2014; 43:649–653.
Article32. Chung EB, Enzinger FM. Infantile myofibromatosis. Cancer. 1981; 48:1807–1818.
Article33. Sargar KM, Sheybani EF, Shenoy A, Aranake-Chrisinger J, Khanna G. Pediatric fibroblastic and myofibroblastic tumors: a pictorial review. Radiographics. 2016; 36:1195–1214.
Article34. Gruber H, Glodny B, Bendix N, Tzankov A, Peer S. High-resolution ultrasound of peripheral neurogenic tumors. Eur Radiol. 2007; 17:2880–2888.
Article35. Reynolds DL Jr, Jacobson JA, Inampudi P, Jamadar DA, Ebrahim FS, Hayes CW. Sonographic characteristics of peripheral nerve sheath tumors. AJR Am J Roentgenol. 2004; 182:741–744.
Article36. Gosein M, Ameeral A, Banfield R, Mosodeen M. Plexiform neurofibroma of the wrist: imaging features and when to suspect malignancy. Case Rep Radiol. 2013; 2013:493752.
Article37. Quinn TJ, Jacobson JA, Craig JG, van Holsbeeck MT. Sonography of Morton's neuromas. AJR Am J Roentgenol. 2000; 174:1723–1728.
Article38. Wasa J, Nishida Y, Tsukushi S, Shido Y, Sugiura H, Nakashima H, et al. MRI features in the differentiation of malignant peripheral nerve sheath tumors and neurofibromas. AJR Am J Roentgenol. 2010; 194:1568–1574.
Article39. Bhargava R, Parham DM, Lasater OE, Chari RS, Chen G, Fletcher BD. MR imaging differentiation of benign and malignant peripheral nerve sheath tumors: use of the target sign. Pediatr Radiol. 1997; 27:124–129.
Article40. Bensaid B, Giammarile F, Mognetti T, Galoisy-Guibal L, Pinson S, Drouet A, et al. [Utility of 18 FDG positon emission tomography in detection of sarcomatous transformation in neurofibromatosis type 1]. Ann Dermatol Venereol. 2007; 134(10 Pt 1):735–741.41. Wagner JM, Rebik K, Spicer PJ. Ultrasound of soft tissue masses and fluid collections. Radiol Clin North Am. 2019; 57:657–669.
Article42. Kim HK, Kim SM, Lee SH, Racadio JM, Shin MJ. Subcutaneous epidermal inclusion cysts: ultrasound (US) and MR imaging findings. Skeletal Radiol. 2011; 40:1415–1419.
Article43. Yuan WH, Hsu HC, Lai YC, Chou YH, Li AF. Differences in sonographic features of ruptured and unruptured epidermal cysts. J Ultrasound Med. 2012; 31:265–272.
Article44. Hwang JY, Lee SW, Lee SM. The common ultrasonographic features of pilomatricoma. J Ultrasound Med. 2005; 24:1397–1402.
Article45. Eutsler EP, Siegel MJ. Musculoskeletal system and vascular imaging. In : Siegel MJ, editor. Pediatric sonography. 5th ed. Philadelphia, PA: Wolters Kluwer;2018. p. 627.46. Teefey SA, Dahiya N, Middleton WD, Gelberman RH, Boyer MI. Ganglia of the hand and wrist: a sonographic analysis. AJR Am J Roentgenol. 2008; 191:716–720.
Article47. Wang G, Jacobson JA, Feng FY, Girish G, Caoili EM, Brandon C. Sonography of wrist ganglion cysts: variable and noncystic appearances. J Ultrasound Med. 2007; 26:1323–1328. quiz 1330–1331.48. Van Rijn RR, Wilde JC, Bras J, Oldenburger F, McHugh KM, Merks JH. Imaging findings in noncraniofacial childhood rhabdomyosarcoma. Pediatr Radiol. 2008; 38:617–634.
Article49. Miller RW, Young JL Jr, Novakovic B. Childhood cancer. Cancer. 1995; 75:1 Suppl. 395–405.
Article50. Bakri A, Shinagare AB, Krajewski KM, Howard SA, Jagannathan JP, Hornick JL, et al. Synovial sarcoma: imaging features of common and uncommon primary sites, metastatic patterns, and treatment response. AJR Am J Roentgenol. 2012; 199:W208–W215.
Article51. Murphey MD, Gibson MS, Jennings BT, Crespo-Rodríguez AM, Fanburg-Smith J, Gajewski DA. From the archives of the AFIP: imaging of synovial sarcoma with radiologic-pathologic correlation. Radiographics. 2006; 26:1543–1565.52. Kang BS, Choi SH, Cha HJ, Jung YK, Lee JH, Jeong AK, et al. Subcutaneous panniculitis-like T-cell lymphoma: US and CT findings in three patients. Skeletal Radiol. 2007; 36 Suppl 1:S67–S71.
Article53. Bakst RL, Tallman MS, Douer D, Yahalom J. How I treat extramedullary acute myeloid leukemia. Blood. 2011; 118:3785–3793.
Article54. Isaacs H Jr. Cutaneous metastases in neonates: a review. Pediatr Dermatol. 2011; 28:85–93.
Article55. Hanna SL, Kaste S, Jenkins JJ, Hewan-Lowe K, Spence JV, Gupta M, et al. Epithelioid sarcoma: clinical, MR imaging and pathologic findings. Skeletal Radiol. 2002; 31:400–412.56. Giovagnorio F, Valentini C, Paonessa A. High-resolution and color doppler sonography in the evaluation of skin metastases. J Ultrasound Med. 2003; 22:1017–1022. quiz 1023–1025.
Article57. Sood S, Baheti AD, Shinagare AB, Jagannathan JP, Hornick JL, Ramaiya NH, et al. Imaging features of primary and metastatic alveolar soft part sarcoma: single institute experience in 25 patients. Br J Radiol. 2014; 87:20130719.
Article58. Chung HW, Cho KH. Ultrasonography of soft tissue “oops lesions”. Ultrasonography. 2015; 34:217–225.
Article59. Calleja M, Dimigen M, Saifuddin A. MRI of superficial soft tissue masses: analysis of features useful in distinguishing between benign and malignant lesions. Skeletal Radiol. 2012; 41:1517–1524.
Article