J Korean Med Assoc.
2020 Feb;63(2):128-134. 10.5124/jkma.2020.63.2.128.
Updates of adult immunization in Korea
- Affiliations
-
- 1Department of Family Medicine, Myongji Hospital, Hanyang University College of Medicine, Goyang, Korea.
- 2Department of Family Medicine, Soonchunhyang University Seoul Hospital, Seoul, Korea. dryoo@schmc.ac.kr
- KMID: 2470068
- DOI: http://doi.org/10.5124/jkma.2020.63.2.128
Abstract
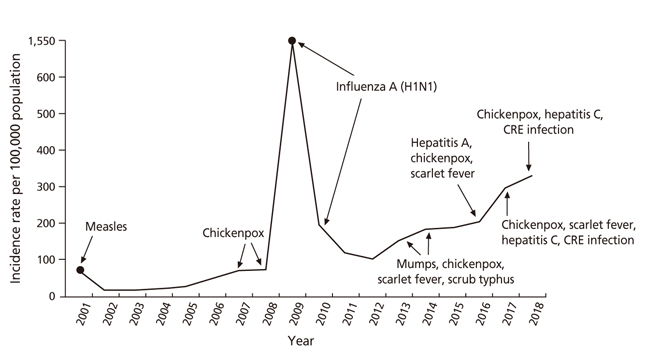
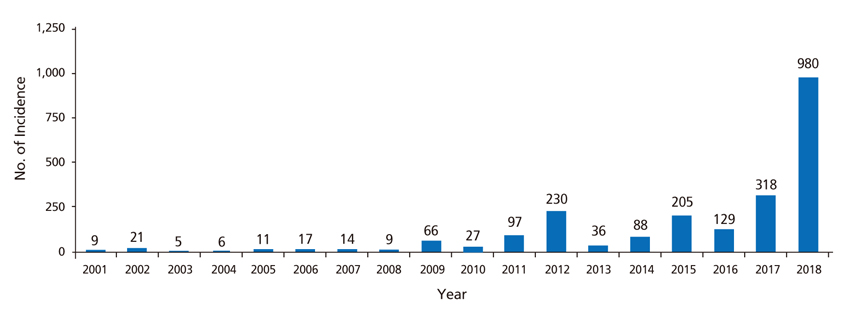
- An increase in the number of patients with infectious diseases in Korea, can be attributed to various factors, such as the prevalence of new infectious diseases of the 21st century, the re-emergence of past infectious diseases, an increase in the number of elderly individuals, patients with chronic diseases, immune deficiency, and globalization. In this context, vaccination becomes vital for the adult population. Although, the guidelines for adult immunization are currently being updated, the rate of adult vaccination remains lower than that of infant vaccination. At present, the major challenges for increasing the rate of adult immunization include negative views on the need for some immunizations and a lack of understanding of group immunity among the youth. Consequently, a successful immunization program will be required to direct efforts towards educating patients and spreading awareness. Based on the current guidelines and practical applications, varicella zoster; Japanese encephalitis; tetanus, diphtheria, and pertussis; pneumococcus; measles, mumps, and rubella; and hepatitis A vaccines could effectively be considered for adult vaccination.
MeSH Terms
-
Adolescent
Adult*
Aged
Chickenpox
Chronic Disease
Communicable Diseases
Diphtheria
Encephalitis, Japanese
Hepatitis A Vaccines
Herpes Zoster
Humans
Immunization Programs
Immunization*
Infant
Internationality
Korea*
Measles
Mumps
Pneumococcal Vaccines
Prevalence
Rubella
Streptococcus pneumoniae
Tetanus
Vaccination
Whooping Cough
Hepatitis A Vaccines
Pneumococcal Vaccines
Figure
Reference
-
1. Bloom DE, Cadarette D. Infectious disease threats in the twenty-first century: strengthening the global response. Front Immunol. 2019; 10:549.
Article2. Korea Centers for Disease Control and Prevention. Infectious disease surveillance yearbook 2018 [Internet]. Cheongju: Korea Centers for Disease Control and Prevention;2019. cited 2019 Dec 24. Available from: http://www.cdc.go.kr/npt/biz/npp/portal/nppPblctDtaView.do?pblctDtaSeAt=1&pblctDtaSn=1873.3. Kim JH. Recent trends in anti-vaccine movement and future directions. Korean J Pediatr Infect Dis. 2007; 14:37–42.
Article4. Kovaiou RD, Herndler-Brandstetter D, Grubeck-Loebenstein B. Age-related changes in immunity: implications for vaccination in the elderly. Expert Rev Mol Med. 2007; 9:1–17.
Article5. Oh MD, Lee JK. Milestones in history of adult vaccination in Korea. Clin Exp Vaccine Res. 2012; 1:9–17.
Article6. Korean Society of Infectious Diseases. Guideline for adult immunization 2019 [Internet]. Seoul: Korean Society of Infectious Disease;2019. cited 2019 Dec 24. Available from: http://www.ksid.or.kr/data/sub07.html.7. Korea Centers for Disease Control and Prevention. Guideline for adult immunization 2018 [Internet]. Cheongju: Korea Centers for Disease Control and Prevention;2018. cited 2019 Dec 24. Available from: http://www.cdc.go.kr/CDC/cms/content/mobile/57/142157_view.html.8. Schmader K, Gnann JW Jr, Watson CP. The epidemiological, clinical, and pathological rationale for the herpes zoster vaccine. J Infect Dis. 2008; 197 Suppl 2:S207–S215.
Article9. Oxman MN, Levin MJ. Shingles Prevention Study Group. Vaccination against herpes zoster and postherpetic neuralgia. J Infect Dis. 2008; 197 Suppl 2:S228–S236.
Article10. Public Health England. Impact of shingles vaccination programme confirmed [Internet]. London: Public Health England;2018. cited 2019 Dec 24. Available from: https://www.gov.uk/government/publications/health-protection-report-volume-12-2018/hpr-volume-12-issue-1-news-5-january.11. Dooling KL, Guo A, Patel M, Lee GM, Moore K, Belongia EA, Harpaz R. Recommendations of the Advisory Committee on Immunization Practices for use of herpes zoster vaccines. MMWR Morb Mortal Wkly Rep. 2018; 67:103–108.
Article12. Yoo SM. Cost-effectiveness analysis of vaccination against herpes zoster in older adults aged over 60 years in Korea [dissertation]. Seoul: Seoul National University;2014.13. Korea Centers for Disease Control and Prevention. Web statistics system for infectious disease [Internet]. Cheongju: Korea Centers for Disease Control and Prevention;2019. cited 2019 Dec 24. Available from: https://is.cdc.go.kr/.14. Lee HY, Park YJ, Park O. Incidence and epidemiology of Japanese encephalitis in Korea. Public Health Wkly Rep KCDC. 2015; 8:433–436.15. Chokephaibulkit K, Houillon G, Feroldi E, Bouckenooghe A. Safety and immunogenicity of a live attenuated Japanese encephalitis chimeric virus vaccine (IMOJEV) in children. Expert Rev Vaccines. 2016; 15:153–166.
Article16. Jeong CW, Park YJ. Status of pertussis incidences in Korea, 2012. Public Health Wkly Rep. 2013; 6:569–573.17. Korea Centers for Disease Control and Prevention. Pertussis [Internet]. Cheongju: Korea Centers for Disease Control and Prevention;2019. cited 2019 Dec 24. Available from: http://www.cdc.go.kr/npt/biz/npp/ist/bass/bassDissStatsMain.do.18. Status of pertussis incidences in Korea, 2018. Public Health Wkly Rep. 2018; 29:1–2.19. Kwon HJ, Yum SK, Choi UY, Lee SY, Kim JH, Kang JH. Infant pertussis and household transmission in Korea. J Korean Med Sci. 2012; 27:1547–1551.
Article20. Statistics Korea. Cause of death statistics in 2018 [Internet]. Daejeon: Statistics Korea;2019. cited 2019 Dec 24. Available from: http://kostat.go.kr/portal/korea/kor_nw/1/6/2/index.board?bmode=read&aSeq=377606&pageNo=&rowNum=10&amSeq=&sTarget=&sTxt=.21. Tomczyk S, Bennett NM, Stoecker C, Gierke R, Moore MR, Whitney CG, Hadler S, Pilishvili T. Centers for Disease Control and Prevention. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥65 years: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2014; 63:822–825.22. Song JY, Cheong HJ. Pneumococcal vaccine. J Korean Med Assoc. 2014; 57:780–788.
Article23. Choi WS, Choi JH, Kwon KT, Seo K, Kim MA, Lee SO, Hong YJ, Lee JS, Song JY, Bang JH, Choi HJ, Choi YH, Lee DG, Cheong HJ. the Committee of Adult Immunization, the Korean Society of Infectious Diseases. Revised Adult Immunization Guideline recommended by the Korean Society of Infectious Diseases, 2014. Infect Chemother. 2015; 47:68–79.
Article