Clin Exp Vaccine Res.
2012 Jul;1(1):9-17. 10.7774/cevr.2012.1.1.9.
Milestones in history of adult vaccination in Korea
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea.
- 2Department of Family Medicine, Seoul National University College of Medicine, Seoul, Korea. docmohw@snu.ac.kr
- KMID: 2278785
- DOI: http://doi.org/10.7774/cevr.2012.1.1.9
Abstract
- Vaccination is one of the most effective and cost-benefit interventions that reduced the mortality. Major vaccine preventable diseases have decreased dramatically after the introduction of immunization program in Korea. In this article, we review milestones in history of immunization program, especially in adult vaccination.
Keyword
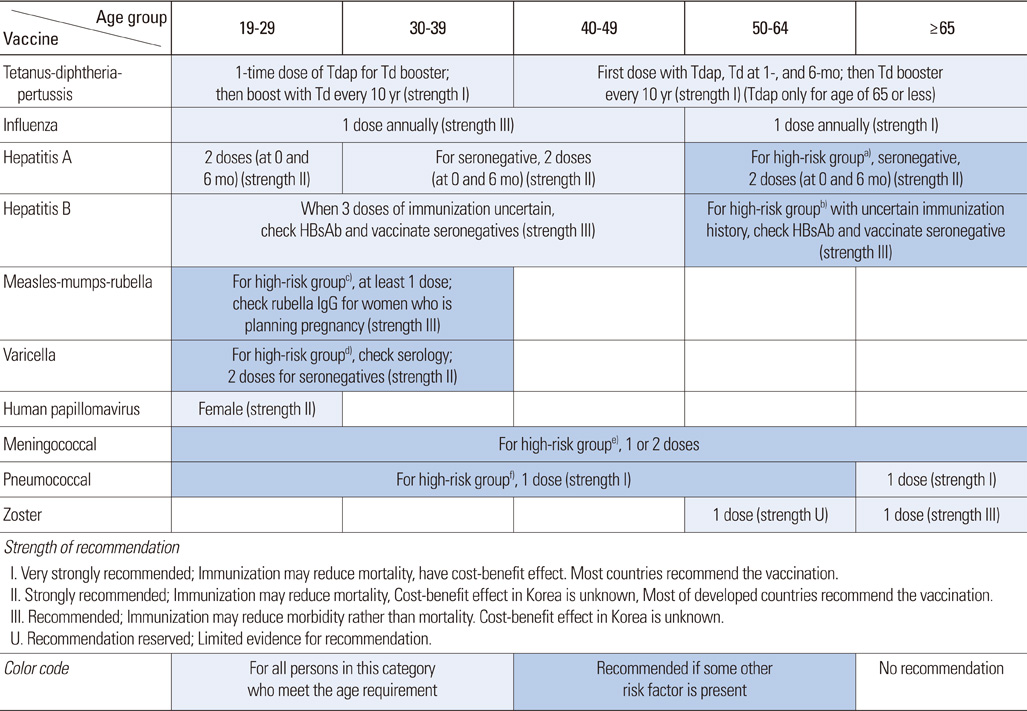
Figure
Cited by 4 articles
-
Leptospirosis in the Republic of Korea: Historical Perspectives, Current Status and Future Challenges
Min Ja Kim
Infect Chemother. 2013;45(2):137-144. doi: 10.3947/ic.2013.45.2.137.Investigation of a Mumps Outbreak in a Dental Clinic at a University Hospital
Jeong Eun Lee, Soon Ok Lee, Jin Suk Kang, Jongyoun Yi, Kye-Hyung Kim
Infect Chemother. 2019;51(3):256-262. doi: 10.3947/ic.2019.51.3.256.Updates of adult immunization in Korea
Hyun-Young Shin, Byung Wook Yoo
J Korean Med Assoc. 2020;63(2):128-134. doi: 10.5124/jkma.2020.63.2.128.Recommended immunization schedule for adults in Korea, by the Korean Society of Infectious Diseases, 2012
Clin Exp Vaccine Res. 2014;3(1):110-112. doi: 10.7774/cevr.2014.3.1.110.
Reference
-
1. Lee JK, Choi WS. Immunization policy in Korea. Infect Chemother. 2008. 40:14–23.
Article2. Korea Centers for Disease Control and Prevention. National immunization program. Mon Newsl Natl Immun Program. 2007. 5:117–118.3. Korea Centers for Disease Control and Prevention. Epidemiology and prevention of vaccine-preventable diseases. 2011. Seoul: Korea Centers for Disease Control and Prevention.4. Chun CH. Overview of acute communicable diseases in Korea. 1975. Seoul: Newest Medicine Co.;85–88.5. Korean Society of Infectious Diseases. History of communicable diseases in Korea. 2009. Seoul: Kunja Publishing Co.;319–410.6. Kim SH, Yeo SG, Jang HC, et al. Clinical responses to smallpox vaccine in vaccinia-naive and previously vaccinated populations: undiluted and diluted Lancy-Vaxina vaccine in a single-blind, randomized, prospective trial. J Infect Dis. 2005. 192:1066–1070.
Article7. Jang HC, Kim CJ, Kim KH, et al. A randomized, double-blind, controlled clinical trial to evaluate the efficacy and safety of CJ-50300, a newly developed cell culture-derived smallpox vaccine, in healthy volunteers. Vaccine. 2010. 28:5845–5849.
Article8. Lee HW, Lee PW, Johnson KM. Isolation of the etiologic agent of Korean hemorrhagic fever. J Infect Dis. 1978. 137:298–308.
Article9. French GR, Foulke RS, Brand OA, Eddy GA, Lee HW, Lee PW. Korean hemorrhagic fever: propagation of the etiologic agent in a cell line of human origin. Science. 1981. 211:1046–1048.
Article10. Lee HW, Ahn CN. Development of inactivated vaccine against hemorrhagic fever with renal syndrome. J Korean Soc Virol. 1988. 18:143–148.11. Chu YK, Gligic A, Tomanovic S, et al. A field efficacy trial of inactivated Hantaan virus vaccine (Hantavax (TM)) against hemorrhagic fever with renal syndrome (HFRS) in the endemic areas of Yugoslavia from 1996 to 1998. J Korean Soc Virol. 1999. 29:55–64.12. Park K, Kim CS, Moon KT. Protective effectiveness of hantavirus vaccine. Emerg Infect Dis. 2004. 10:2218–2220.
Article13. Sohn YM, Roh HO, Kim HS. Neutralizing antibody response to two doses of formalin inactivated mouse brain-derived Hantaan virus vaccine (HantavaxR) in healthy adults. Korean J Infect Dis. 1998. 30:325–331.14. Chu YK, Woo YD, Lee HW. Immune response and antibody persistence against Hantaan virus of vaccinees with Hantavax (TM). Korean J Infect Dis. 1998. 30:317–324.15. Cho HW, Howard CR. Antibody responses in humans to an inactivated hantavirus vaccine (Hantavax). Vaccine. 1999. 17:2569–2575.
Article16. Woo YD, Chu YK, Baek LJ, Lee HW. An immunoserological study of vaccine against haemorrhagic fever with renal syndrome. J Korean Soc Virol. 2000. 30:11–18.17. Sohn YM, Rho HO, Park MS, Kim JS, Summers PL. Primary humoral immune responses to formalin inactivated hemorrhagic fever with renal syndrome vaccine (Hantavax): consideration of active immunization in South Korea. Yonsei Med J. 2001. 42:278–284.
Article18. Cho HW, Howard CR, Lee HW. Review of an inactivated vaccine against hantaviruses. Intervirology. 2002. 45:328–333.
Article19. Lee HW, Baek LJ, Woo YD. The persistence of immunity against hemorrhagic fever with renal syndrome among Hantaan virus vaccinees. J Korean Soc Microbiol. 1992. 27:73–77.20. Shinozaki F, Sada E, Tamai T, Kobayashi Y. Characterization of Leptospira strains HY-1, HY-2, and HY-10 isolated in Korea by means of monoclonal antibodies and restriction endonuclease DNA analysis. Am J Trop Med Hyg. 1992. 46:342–349.
Article21. Kim MH, Park SC. Korean Society of Infectious Diseases. Leptospirosis. Vaccination for adult. 2012. 2nd ed. Seoul: MIP;225–235.22. Kim YT. Current situation and elimination plan of influenza. J Korean Med Assoc. 2004. 47:1116–1128.
Article23. Korea Centers for Disease Cntrol and Prevention. Analysis of reported pandemic influenza (A/H1N1 2009) virus infections in Korea: from April, 2009 through August, 2010. Public Health Wkly Rep. 2010. 3:637–642.24. Oh CE, Lee J, Kang JH, et al. Safety and immunogenicity of an inactivated split-virus influenza A/H1N1 vaccine in healthy children from 6 months to <18 years of age: a prospective, open-label, multi-center trial. Vaccine. 2010. 28:5857–5863.
Article25. Cheong HJ, Song JY, Heo JY, et al. Immunogenicity and safety of the influenza A/H1N1 2009 inactivated split-virus vaccine in young and older adults: MF59-adjuvanted vaccine versus nonadjuvanted vaccine. Clin Vaccine Immunol. 2011. 18:1358–1364.
Article26. Lee YK, Kwon Y, Kim DW, et al. 2009-2010 novel influenza A (H1N1) vaccination coverage in the Republic of Korea. Am J Infect Control. 2012. 40:481–483.
Article27. Oh MD. Korean Society of Infectious Diseases. Adult vaccination: Why we need it? Vaccination for adult. 2012. 2nd ed. Seoul: MIP;2–6.28. Lim J, Eom CS, Kim S, Ke S, Cho B. Pneumococcal vaccination rate among elderly in South Korea. J Korean Geriatr Soc. 2010. 14:18–24.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Infectious diseases and medical institutions in the late Chosen dynasty
- Knowledge, Health Belief, and Vaccination Behavior on Hepatitis A among University Students
- Hepatitis B Vaccination Coverage and Related Factors among Aged 19 or Older in Republic of Korea
- Introduction of the modern western medicine in late Choson period (I)
- Smallpox Epidemics and Folk's Responses in the late Chosun Period