Korean J Gastroenterol.
2019 Dec;74(6):349-355. 10.4166/kjg.2019.74.6.349.
Mixed Neuroendocrine-non-neuroendocrine Neoplasm of the Stomach that is Distributed in Depth on the Same Tumor: Inconsistent with the Definition of Mixed Adenoneuroendocrine Carcinoma in the 2010 World Health Organization Classification of Tumors of the Digestive System
- Affiliations
-
- 1Department of Internal Medicine, Kosin University College of Medicine, Busan, Korea. forjkw@gmail.com
- KMID: 2469910
- DOI: http://doi.org/10.4166/kjg.2019.74.6.349
Abstract
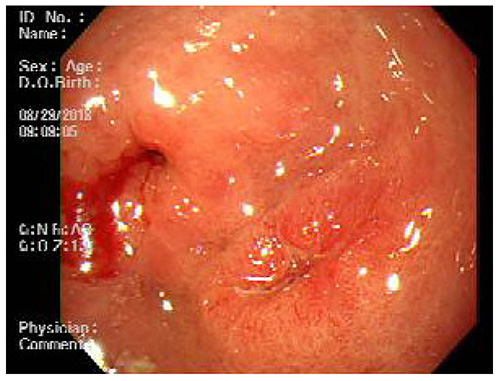
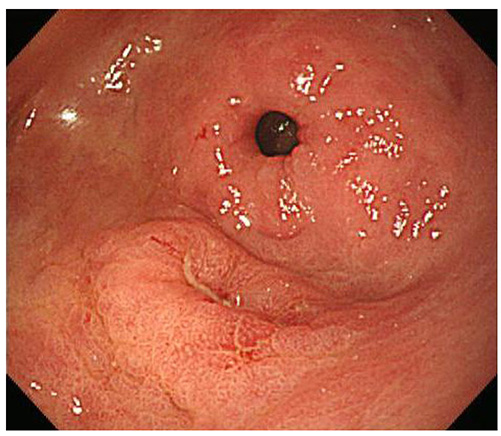
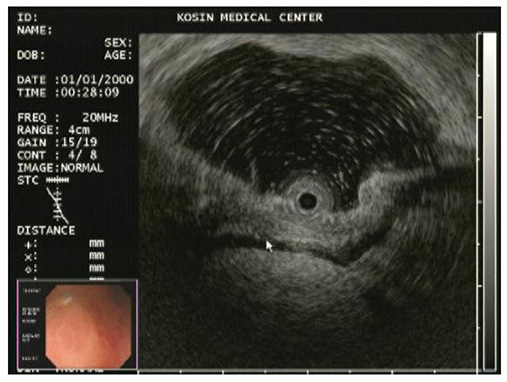
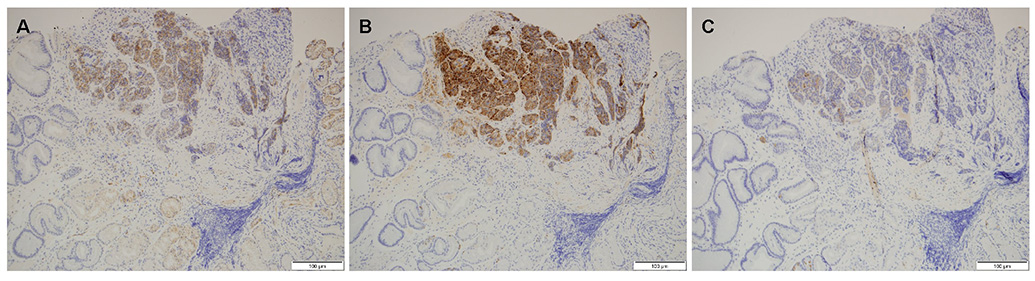
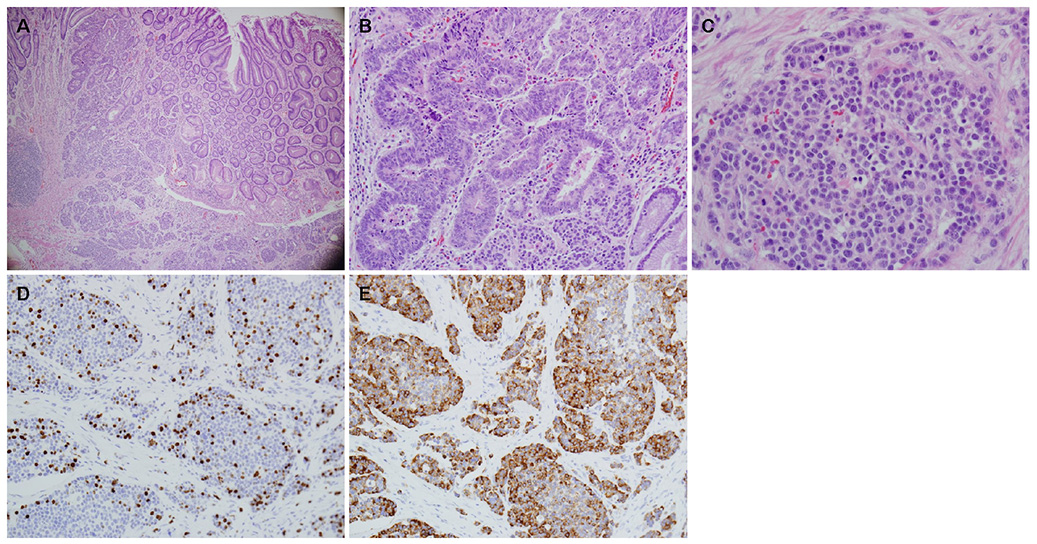
- A mixed adenoneuroendocrine carcinoma (MANEC) of the stomach is a rare disease entity that was first defined by the World Health Organization (WHO) classification (2010) for tumors of the digestive system. According to the WHO classification (2010), MANEC is referred to as a tumor with both neuroendocrine and non-neuroendocrine neoplasms; each component of the tumor should be at least 30%. On the other hand, this cut-off value lacks clinical evidence and does not explain the characteristics and heterogeneity of this tumor. A 66-year-old male diagnosed with early gastric cancer (EGC) at a community hospital was referred to the Kosin University Gospel Hospital for further evaluation of gastric cancer. Esophagogastroduodenoscopy and EUS performed at the Kosin University Gospel Hospital revealed a sub-mucosal tumor-like component. In addition, a re-biopsy revealed a neuroendorine tumor at different depths of the same tumor. The final pathologic-diagnosis through surgery revealed a mixed neuroendocrine-non-neuroendocrine neoplasm, which is inconsistent with the definition of MANEC. Clinicians should consider EUS when a tumor has atypical endoscopic findings, even if EGC has already been diagnosed.
MeSH Terms
Figure
Reference
-
1. Cordier R. Les cellules argentaffines dans les tumeurs intestinales. Arch Int Med Exp. 1924; 1:59–63.2. Lewin K. Carcinoid tumors and the mixed (composite) glandular-endocrine cell carcinomas. Am J Surg Pathol. 1987; 11 Suppl 1:71–86.3. Rindi G, Petrone G, Inzani F. The 2010 WHO classification of digestive neuroendocrine neoplasms: a critical appraisal four years after its introduction. Endocr Pathol. 2014; 25:186–192.4. La Rosa S, Sessa F, Uccella S. Mixed neuroendocrine-nonneuroendocrine neoplasms (MiNENs): unifying the concept of a heterogeneous group of neoplasms. Endocr Pathol. 2016; 27:284–311.5. Pham QD, Mori I, Osamura RY. A case report: gastric mixed neuroendocrine-nonneuroendocrine neoplasm with aggressive neuroendocrine component. Case Rep Pathol. 2017; 2017:9871687.6. Wang YH, Lin Y, Xue L, Wang JH, Chen MH, Chen J. Relationship between clinical characteristics and survival of gastroenteropancreatic neuroendocrine neoplasms: a single-institution analysis (1995–2012) in South China. BMC Endocr Disord. 2012; 12:30.7. Gurzu S, Kadar Z, Bara T, et al. Mixed adenoneuroendocrine carcinoma of gastrointestinal tract: report of two cases. World J Gastroenterol. 2015; 21:1329–1333.8. Kim KH, Lee HJ, Lee SH, Hwang SH. Mixed adenoneuroendocrine carcinoma in the stomach: a case report with a literature review. Ann Surg Treat Res. 2018; 94:270–273.9. La Rosa S, Marando A, Sessa F, Capella C. Mixed adenoneuroendocrine carcinomas (MANECs) of the gastrointestinal tract: an update. Cancers (Basel). 2012; 4:11–30.10. Wu C, Bao W, Rao Q, et al. Clinicopathological features and prognosis of gastric mixed adenoneuroendocrine carcinoma. Int J Clin Exp Pathol. 2018; 11:1499–1509.11. Kim JJ, Kim JY, Hur H, Cho YK, Han SU. Clinicopathologic significance of gastric adenocarcinoma with neuroendocrine features. J Gastric Cancer. 2011; 11:195–199.12. Xu TM, Wang CS, Jia CW, Qian JM, Li JN. Clinicopathological features of primary gastric neuroendocrine neoplasms: a single-center analysis. J Dig Dis. 2016; 17:162–168.13. Pericleous M, Toumpanakis C, Lumgair H, et al. Gastric mixed adenoneuroendocrine carcinoma with a trilineage cell differentiation: case report and review of the literature. Case Rep Oncol. 2012; 5:313–319.14. Kim TY, Chae HD. Composite neuroendocrine carcinoma with adenocarcinoma of the stomach misdiagnosed as a giant submucosal tumor. J Gastric Cancer. 2011; 11:126–130.15. Ito H, Kudo A, Matsumura S, et al. Mixed adenoneuroendocrine carcinoma of the colon progressed rapidly after hepatic rupture: report of a case. Int Surg. 2014; 99:40–44.16. La Rosa S, Inzani F, Vanoli A, et al. Histologic characterization and improved prognostic evaluation of 209 gastric neuroendocrine neoplasms. Hum Pathol. 2011; 42:1373–1384.17. Shen C, Chen H, Chen H, et al. Surgical treatment and prognosis of gastric neuroendocrine neoplasms: a single-center experience. BMC Gastroenterol. 2016; 16:111.18. Milione M, Maisonneuve P, Pellegrinelli A, et al. Ki67 proliferative index of the neuroendocrine component drives MANEC prognosis. Endocr Relat Cancer. 2018; 25:583–593.19. Lee JH, Kim HW, Kang DH, Choi CW, Park SB, Kim SH. A gastric composite tumor with an adenocarcinoma and a neuroendocrine carcinoma: a case report. Clin Endosc. 2013; 46:280–283.20. Yang G, Li D, Zheng F, Yang L. Long-term disease free survival of gastric mixed adenoneuroendocrine carcinoma treated with multimodality therapy: a case report. Mol Clin Oncol. 2018; 8:653–656.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Correction: Mixed Neuroendocrine-non-neuroendocrine Neoplasm of the Stomach that is Distributed in Depth on the Same Tumor: Inconsistent with the Definition of Mixed Adenoneuroendocrine Carcinoma in the 2010 World Health Organization Classification of Tumors of the Digestive System
- Mixed adenoneuroendocrine carcinoma of the ampulla of Vater: Three case reports and a literature review
- Gastric Mixed Adenoneuroendocrine Carcinoma with Revised Diagnosis after Retrospective Pathologic Review
- Composite Neuroendocrine Carcinoma with Adenocarcinoma of the Stomach Misdiagnosed as a Giant Submucosal Tumor
- Imaging Findings of Neuroendocrine Neoplasm in Biliary Duct with Liver Metastasis