Korean J Transplant.
2019 Dec;33(4):153-158. 10.4285/jkstn.2019.33.4.153.
Successful treatment of early acute antibody-mediated rejection in an human leukocyte antigen-incompatible and ABO-incompatible living-donor kidney transplant patient
- Affiliations
-
- 1Department of Surgery, Seoul National University Hospital, Seoul, Korea. jcyjs@snu.ac.kr
- 2Transplantation Center, Seoul National University Hospital, Seoul, Korea.
- 3Transplantation Research Institute, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 2468166
- DOI: http://doi.org/10.4285/jkstn.2019.33.4.153
Abstract
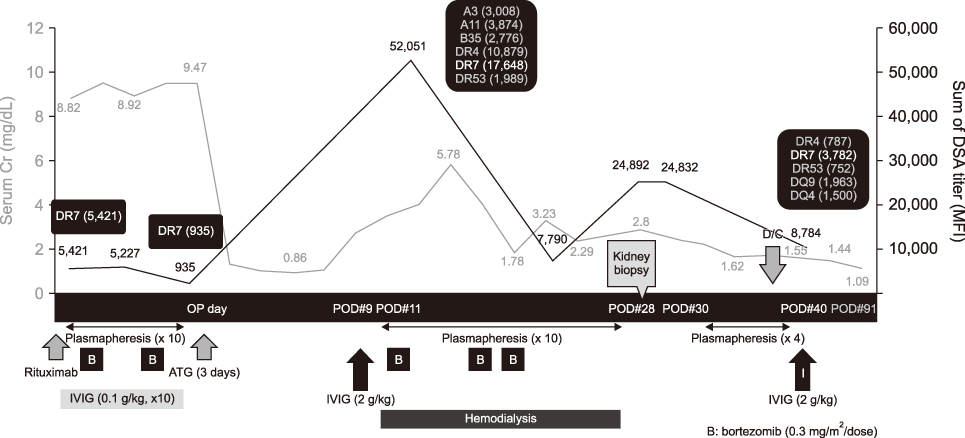
- For successful human leukocyte antigen-incompatible (HLAi) or ABO-incompatible (ABOi) living-donor kidney transplantations (LDKTs), pretransplant desensitization is essential; however, early antibody-mediated rejection (ABMR) remains the most important complication after HLAi or ABOi transplantation. Here, we report a case of early acute ABMR in simultaneous HLAi and ABOi LDKT with preformed donor-specific antibody (DSA), despite desensitization. Dialysis-dependent, severe ABMR occurred with a rebound of pre-existing DSA and appearance of de novo DSA after initial normalization of renal function, 8 days postoperatively. However, a low anti-ABO antibody titer (1:8) was maintained after transplantation. Combination therapy of plasmapheresis, high-dose intravenous immunoglobulin, and bortezomib improved both ABMR and renal functions. Thus, an appropriate preventive and therapeutic management for early ABMR is important among high-risk LDKT patients. Furthermore, early AMBR can occur despite pretransplant desensitization as seen in this case, and close monitoring of the patient and prompt management are considered vital for better therapeutic outcomes.
MeSH Terms
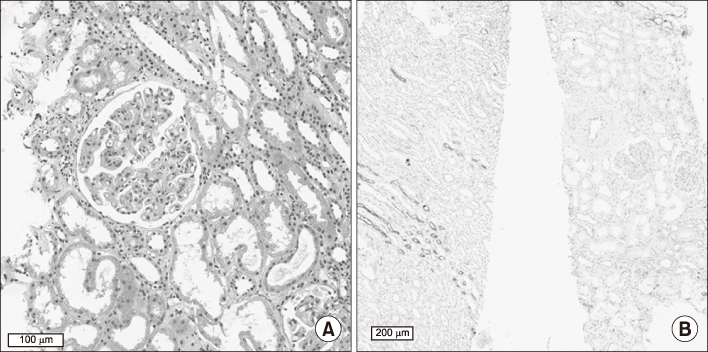
Figure
Cited by 1 articles
-
Clinical significance of de novo donor-specific antibody in kidney transplant recipients with chronic antibody-mediated rejection
Woo Yeong Park, Yaerim Kim, Jin Hyuk Paek, Kyubok Jin, Seungyeup Han
Korean J Transplant. 2021;35(1):33-40. doi: 10.4285/kjt.20.0052.
Reference
-
1. Davis S, Cooper JE. Acute antibody-mediated rejection in kidney transplant recipients. Transplant Rev (Orlando). 2017; 31:47–54.
Article2. Cardinal H, Dieudé M, Hébert MJ. The emerging importance of non-HLA autoantibodies in kidney transplant complications. J Am Soc Nephrol. 2017; 28:400–406.
Article3. Tasaki M, Saito K, Nakagawa Y, Tomita Y, Takahashi K. Ch12. ABO-incompatible kidney transplantation. In : Abdeldayem H, El-Kased AF, El-Shaarawy A, editors. Frontiers in transplantology. London, UK: Rijeka: InTech;2016. p. 285–300.4. Kwun J, Burghuber C, Manook M, Iwakoshi N, Gibby A, Hong JJ, et al. Humoral compensation after bortezomib treatment of allosensitized recipients. J Am Soc Nephrol. 2017; 28:1991–1996.
Article5. Haas M, Loupy A, Lefaucheur C, Roufosse C, Glotz D, Seron D, et al. The Banff 2017 Kidney Meeting Report: revised diagnostic criteria for chronic active T cell-mediated rejection, antibody-mediated rejection, and prospects for integrative endpoints for next-generation clinical trials. Am J Transplant. 2018; 18:293–307.
Article6. Kong JM, Ahn J, Park JB, Chung BH, Yang J, Kim JK, et al. ABO incompatible living donor kidney transplantation in Korea: highly uniform protocols and good medium-term outcome. Clin Transplant. 2013; 27:875–881.
Article7. Korean Organ Transplant Registry. KOTRY annual data report 2018 [Internet]. Seoul: Korean Organ Transplant Registry;2019. cited 2019 Dec 20. Available from: http://www.kotry.org/.8. Orandi BJ, Luo X, Massie AB, Garonzik-Wang JM, Lonze BE, Ahmed R, et al. Survival benefit with kidney transplants from HLA-incompatible live donors. N Engl J Med. 2016; 374:940–950.
Article9. Orandi BJ, Garonzik-Wang JM, Massie AB, Zachary AA, Montgomery JR, Van Arendonk KJ, et al. Quantifying the risk of incompatible kidney transplantation: a multicenter study. Am J Transplant. 2014; 14:1573–1580.
Article10. Orandi BJ, Chow EH, Hsu A, Gupta N, Van Arendonk KJ, Garonzik-Wang JM, et al. Quantifying renal allograft loss following early antibody-mediated rejection. Am J Transplant. 2015; 15:489–498.
Article11. Hart A, Smith JM, Skeans MA, Gustafson SK, Stewart DE, Cherikh WS, et al. OPTN/SRTR 2015 annual data report: kidney. Am J Transplant. 2017; 17 Suppl 1:21–116.
Article12. Schinstock C, Stegall MD. Acute antibody-mediated rejection in renal transplantation: current clinical management. Curr Transplant Rep. 2014; 1:78–85.
Article13. Woodle ES, Shields AR, Ejaz NS, Sadaka B, Girnita A, Walsh RC, et al. Prospective iterative trial of proteasome inhibitor-based desensitization. Am J Transplant. 2015; 15:101–118.
Article14. Haas M, Sis B, Racusen LC, Solez K, Glotz D, Colvin RB, et al. Banff 2013 meeting report: inclusion of c4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am J Transplant. 2014; 14:272–283.
Article15. Burghuber CK, Manook M, Ezekian B, Gibby AC, Leopardi FV, Song M, et al. Dual targeting: combining costimulation blockade and bortezomib to permit kidney transplantation in sensitized recipients. Am J Transplant. 2019; 19:724–736.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- ABO-Incompatible Living Donor Liver Transplantation
- Overcoming high pre-transplant isoagglutinin titers using high-dose intravenous immunoglobulin, salvage plasmapheresis, and booster rituximab without splenectomy in ABO-incompatible living donor liver transplantation: a case report
- Successful Pediatric ABO-Incompatible Kidney Transplantation without Pretransplant Plasmapheresis: Report of a Case
- The Diagnosis of Acute Antibody-Mediated Rejection in ABO-Incompatible Liver Transplants
- Effect of preexisting human leukocyte antigen donor-specific antibodies especially human leukocyte antigen-DQ on kidney transplant outcome