Korean J Radiol.
2017 Feb;18(1):194-207. 10.3348/kjr.2017.18.1.194.
Advanced MRI for Pediatric Brain Tumors with Emphasis on Clinical Benefits
- Affiliations
-
- 1Department of Radiology and Research Institute of Radiology, Asan Medical Center, University of Ulsan College of Medicine, Seoul 05505, Korea. hwgoo@amc.seoul.kr
- 2Department of Neurosurgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul 05505, Korea.
- KMID: 2468133
- DOI: http://doi.org/10.3348/kjr.2017.18.1.194
Abstract
- Conventional anatomic brain MRI is often limited in evaluating pediatric brain tumors, the most common solid tumors and a leading cause of death in children. Advanced brain MRI techniques have great potential to improve diagnostic performance in children with brain tumors and overcome diagnostic pitfalls resulting from diverse tumor pathologies as well as nonspecific or overlapped imaging findings. Advanced MRI techniques used for evaluating pediatric brain tumors include diffusion-weighted imaging, diffusion tensor imaging, functional MRI, perfusion imaging, spectroscopy, susceptibility-weighted imaging, and chemical exchange saturation transfer imaging. Because pediatric brain tumors differ from adult counterparts in various aspects, MRI protocols should be designed to achieve maximal clinical benefits in pediatric brain tumors. In this study, we review advanced MRI techniques and interpretation algorithms for pediatric brain tumors.
Keyword
MeSH Terms
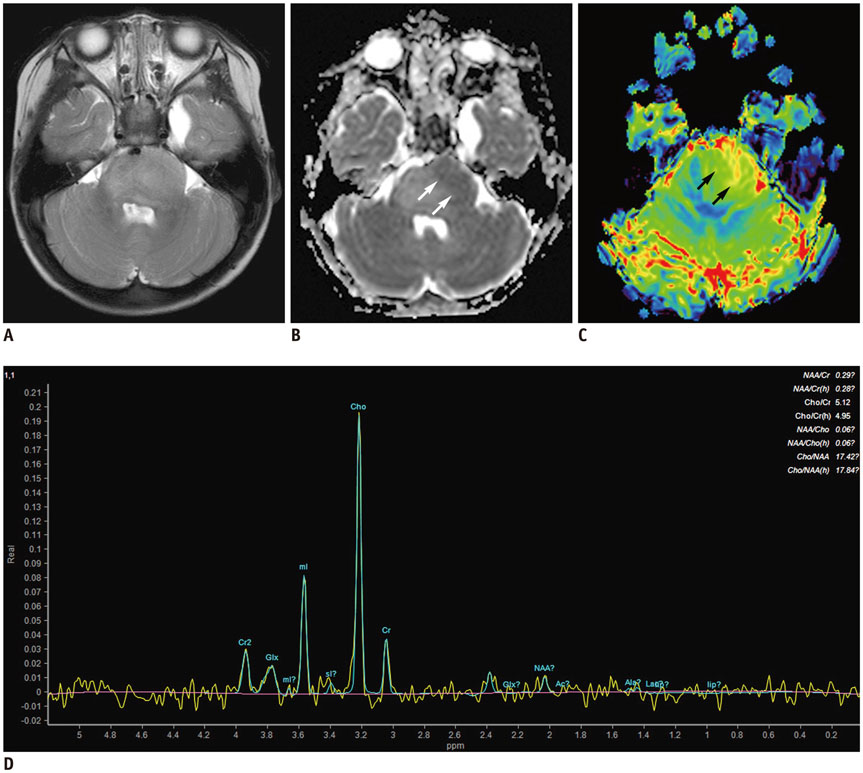
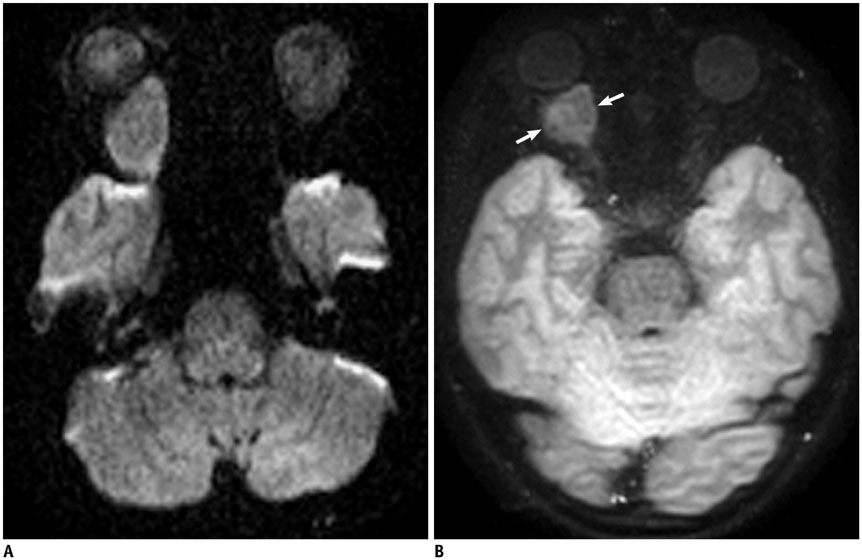
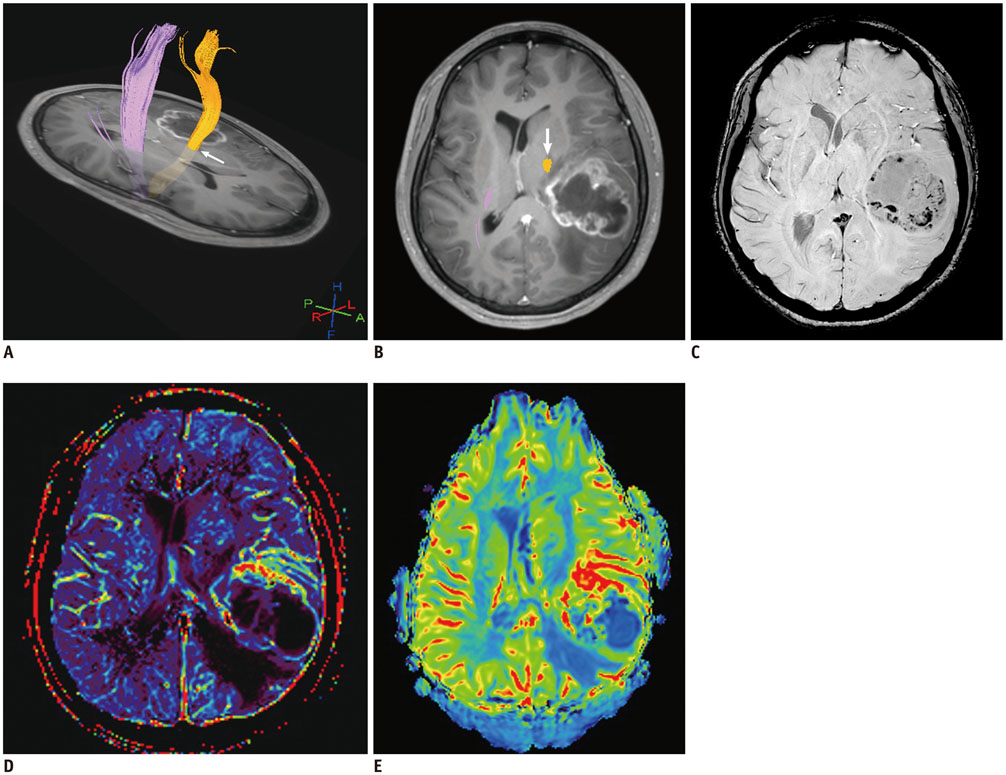
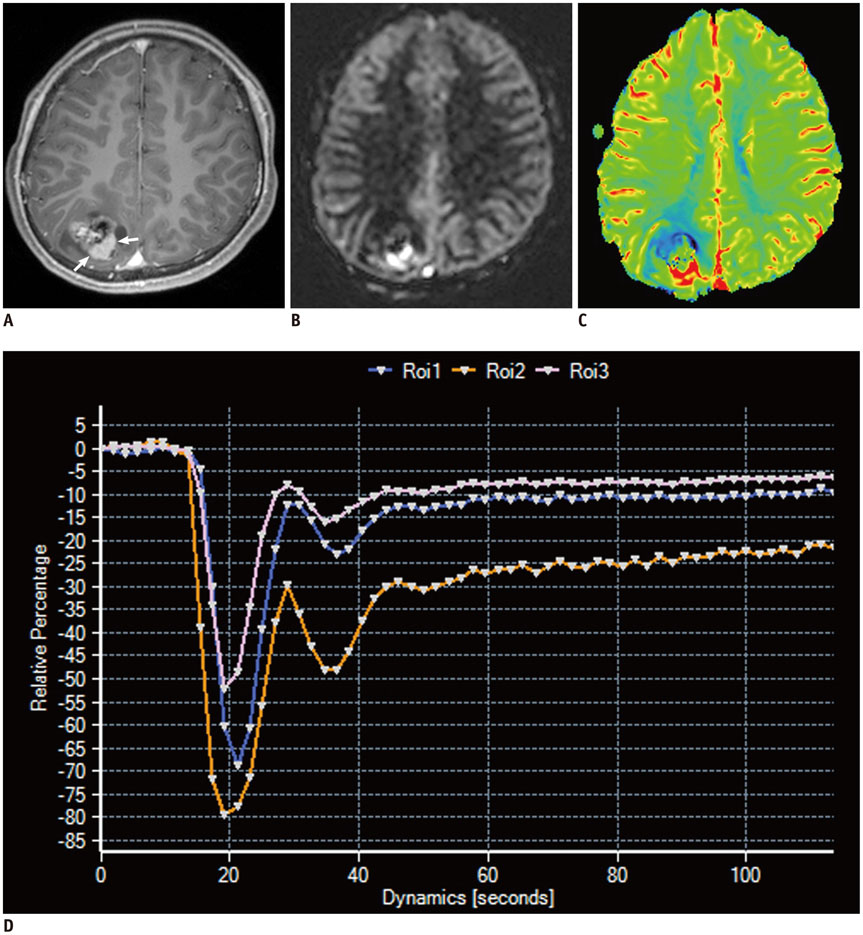
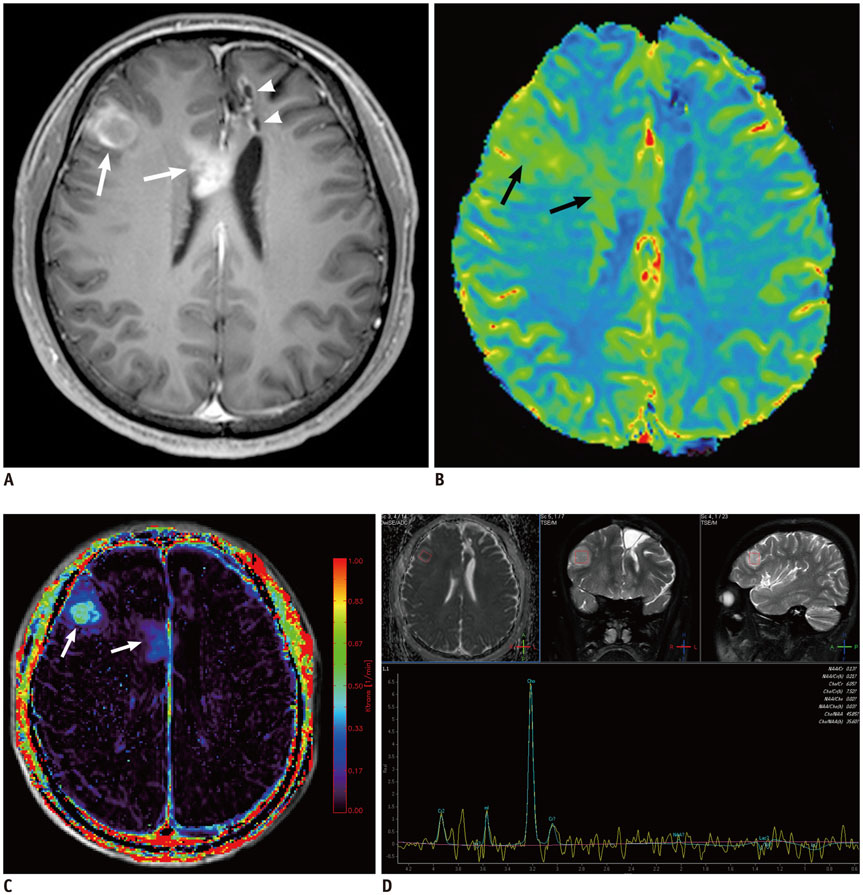
Figure
Cited by 1 articles
-
Quantitative Imaging in Pediatric Hepatobiliary Disease
Haesung Yoon, Hyun Joo Shin, Myung-Joon Kim, Mi-Jung Lee
Korean J Radiol. 2019;20(9):1342-1357. doi: 10.3348/kjr.2019.0002.
Reference
-
1. Barkovich AJ. Pediatric Neuroimaging. 4th ed. Philadelphia, PA: Lippincott Williams & Wilkins;2005.2. Panigrahy A, Blüml S. Neuroimaging of pediatric brain tumors: from basic to advanced magnetic resonance imaging (MRI). J Child Neurol. 2009; 24:1343–1365.3. Radbruch A, Bendszus M. Advanced MR imaging in neurooncology. Clin Neuroradiol. 2015; 25:Suppl 2. 143–149.4. Rossi A, Gandolfo C, Morana G, Severino M, Garrè ML, Cama A. New MR sequences (diffusion, perfusion, spectroscopy) in brain tumours. Pediatr Radiol. 2010; 40:999–1009.5. Poretti A, Meoded A, Huisman TA. Neuroimaging of pediatric posterior fossa tumors including review of the literature. J Magn Reson Imaging. 2012; 35:32–47.6. Lacerda S, Law M. Magnetic resonance perfusion and permeability imaging in brain tumors. Neuroimaging Clin N Am. 2009; 19:527–557.7. Romano A, Rossi Espagnet MC, Calabria LF, Coppola V, Figà Talamanca L, Cipriani V, et al. Clinical applications of dynamic susceptibility contrast perfusion-weighted MR imaging in brain tumours. Radiol Med. 2012; 117:445–460.8. Plaza MJ, Borja MJ, Altman N, Saigal G. Conventional and advanced MRI features of pediatric intracranial tumors: posterior fossa and suprasellar tumors. AJR Am J Roentgenol. 2013; 200:1115–1124.9. Borja MJ, Plaza MJ, Altman N, Saigal G. Conventional and advanced MRI features of pediatric intracranial tumors: supratentorial tumors. AJR Am J Roentgenol. 2013; 200:W483–W503.10. Vinogradov E, Sherry AD, Lenkinski RE. CEST: from basic principles to applications, challenges and opportunities. J Magn Reson. 2013; 229:155–172.11. Goo HW. High field strength magnetic resonance imaging in children. J Korean Med Assoc. 2010; 53:1093–1102.12. Lam WW, Poon WS, Metreweli C. Diffusion MR imaging in glioma: does it have any role in the pre-operation determination of grading of glioma. Clin Radiol. 2002; 57:219–225.13. Porto L, Jurcoane A, Schwabe D, Kieslich M, Hattingen E. Differentiation between high and low grade tumours in paediatric patients by using apparent diffusion coefficients. Eur J Paediatr Neurol. 2013; 17:302–307.14. Gimi B, Cederberg K, Derinkuyu B, Gargan L, Koral KM, Bowers DC, et al. Utility of apparent diffusion coefficient ratios in distinguishing common pediatric cerebellar tumors. Acad Radiol. 2012; 19:794–800.15. Bull JG, Saunders DE, Clark CA. Discrimination of paediatric brain tumours using apparent diffusion coefficient histograms. Eur Radiol. 2012; 22:447–457.16. Bai Y, Lin Y, Tian J, Shi D, Cheng J, Haacke EM, et al. Grading of gliomas by using monoexponential, biexponential, and stretched exponential diffusion-weighted MR imaging and diffusion kurtosis MR imaging. Radiology. 2016; 278:496–504.17. Sui Y, Wang H, Liu G, Damen FW, Wanamaker C, Li Y, et al. Differentiation of low- and high-grade pediatric brain tumors with high b-value diffusion-weighted MR imaging and a fractional order calculus model. Radiology. 2015; 277:489–496.18. Jellison BJ, Field AS, Medow J, Lazar M, Salamat MS, Alexander AL. Diffusion tensor imaging of cerebral white matter: a pictorial review of physics, fiber tract anatomy, and tumor imaging patterns. AJNR Am J Neuroradiol. 2004; 25:356–369.19. Lu S, Ahn D, Johnson G, Law M, Zagzag D, Grossman RI. Diffusion-tensor MR imaging of intracranial neoplasia and associated peritumoral edema: introduction of the tumor infiltration index. Radiology. 2004; 232:221–228.20. Leclercq D, Delmaire C, de Champfleur NM, Chiras J, Lehéricy S. Diffusion tractography: methods, validation and applications in patients with neurosurgical lesions. Neurosurg Clin N Am. 2011; 22:253–268. ix21. Lee SK. Diffusion tensor and perfusion imaging of brain tumors in high-field MR imaging. Neuroimaging Clin N Am. 2012; 22:123–134. ix22. Khong PL, Kwong DL, Chan GC, Sham JS, Chan FL, Ooi GC. Diffusion-tensor imaging for the detection and quantification of treatment-induced white matter injury in children with medulloblastoma: a pilot study. AJNR Am J Neuroradiol. 2003; 24:734–740.23. Fandino J, Kollias SS, Wieser HG, Valavanis A, Yonekawa Y. Intraoperative validation of functional magnetic resonance imaging and cortical reorganization patterns in patients with brain tumors involving the primary motor cortex. J Neurosurg. 1999; 91:238–250.24. O’Shaughnessy ES, Berl MM, Moore EN, Gaillard WD. Pediatric functional magnetic resonance imaging (fMRI): issues and applications. J Child Neurol. 2008; 23:791–801.25. Ogg RJ, Laningham FH, Clarke D, Einhaus S, Zou P, Tobias ME, et al. Passive range of motion functional magnetic resonance imaging localizing sensorimotor cortex in sedated children. J Neurosurg Pediatr. 2009; 4:317–322.26. Kokkonen SM, Nikkinen J, Remes J, Kantola J, Starck T, Haapea M, et al. Preoperative localization of the sensorimotor area using independent component analysis of resting-state fMRI. Magn Reson Imaging. 2009; 27:733–740.27. Jahng GH, Li KL, Ostergaard L, Calamante F. Perfusion magnetic resonance imaging: a comprehensive update on principles and techniques. Korean J Radiol. 2014; 15:554–577.28. Thompson EM, Guillaume DJ, Dósa E, Li X, Nazemi KJ, Gahramanov S, et al. Dual contrast perfusion MRI in a single imaging session for assessment of pediatric brain tumors. J Neurooncol. 2012; 109:105–114.29. Kanda T, Ishii K, Kawaguchi H, Kitajima K, Takenaka D. High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology. 2014; 270:834–841.30. Stojanov D, Aracki-Trenkic A, Benedeto-Stojanov D. Gadolinium deposition within the dentate nucleus and globus pallidus after repeated administrations of gadolinium-based contrast agents-current status. Neuroradiology. 2016; 58:433–441.31. Ho CY, Cardinal JS, Kamer AP, Kralik SF. Relative cerebral blood volume from dynamic susceptibility contrast perfusion in the grading of pediatric primary brain tumors. Neuroradiology. 2015; 57:299–306.32. Cho SK, Na DG, Ryoo JW, Roh HG, Moon CH, Byun HS, et al. Perfusion MR imaging: clinical utility for the differential diagnosis of various brain tumors. Korean J Radiol. 2002; 3:171–179.33. Ho CY, Cardinal JS, Kamer AP, Lin C, Kralik SF. Contrast leakage patterns from dynamic susceptibility contrast perfusion MRI in the grading of primary pediatric brain tumors. AJNR Am J Neuroradiol. 2016; 37:544–551.34. Goo HW, Ra YS. Medullary hemangioblastoma in a child with von Hippel-Lindau disease: vascular tumor perfusion depicted by arterial spin labeling and dynamic contrast-enhanced imaging. J Neurosurg Pediatr. 2015; 16:50–53.35. Hales PW, Phipps KP, Kaur R, Clark CA. A two-stage model for in vivo assessment of brain tumor perfusion and abnormal vascular structure using arterial spin labeling. PLoS One. 2013; 8:e75717.36. White CM, Pope WB, Zaw T, Qiao J, Naeini KM, Lai A, et al. Regional and voxel-wise comparisons of blood flow measurements between dynamic susceptibility contrast magnetic resonance imaging (DSC-MRI) and arterial spin labeling (ASL) in brain tumors. J Neuroimaging. 2014; 24:23–30.37. Yeom KW, Mitchell LA, Lober RM, Barnes PD, Vogel H, Fisher PG, et al. Arterial spin-labeled perfusion of pediatric brain tumors. AJNR Am J Neuroradiol. 2014; 35:395–401.38. Brandão LA, Poussaint TY. Pediatric brain tumors. Neuroimaging Clin N Am. 2013; 23:499–525.39. Wang Z, Sutton LN, Cnaan A, Haselgrove JC, Rorke LB, Zhao H, et al. Proton MR spectroscopy of pediatric cerebellar tumors. AJNR Am J Neuroradiol. 1995; 16:1821–1833.40. Shiroishi MS, Panigrahy A, Moore KR, Nelson MD Jr, Gilles FH, Gonzalez-Gomez I, et al. Combined MRI and MRS improves pre-therapeutic diagnoses of pediatric brain tumors over MRI alone. Neuroradiology. 2015; 57:951–956.41. Löbel U, Sedlacik J, Sabin ND, Kocak M, Broniscer A, Hillenbrand CM, et al. Three-dimensional susceptibility-weighted imaging and two-dimensional T2*-weighted gradient-echo imaging of intratumoral hemorrhages in pediatric diffuse intrinsic pontine glioma. Neuroradiology. 2010; 52:1167–1117.42. Tong KA, Ashwal S, Obenaus A, Nickerson JP, Kido D, Haacke EM. Susceptibility-weighted MR imaging: a review of clinical applications in children. AJNR Am J Neuroradiol. 2008; 29:9–17.43. Di Ieva A, Lam T, Alcaide-Leon P, Bharatha A, Montanera W, Cusimano MD. Magnetic resonance susceptibility weighted imaging in neurosurgery: current applications and future perspectives. J Neurosurg. 2015; 123:1463–1475.44. Togao O, Yoshiura T, Keupp J, Hiwatashi A, Yamashita K, Kikuchi K, et al. Amide proton transfer imaging of adult diffuse gliomas: correlation with histopathological grades. Neuro Oncol. 2014; 16:441–448.45. Sagiyama K, Mashimo T, Togao O, Vemireddy V, Hatanpaa KJ, Maher EA, et al. In vivo chemical exchange saturation transfer imaging allows early detection of a therapeutic response in glioblastoma. Proc Natl Acad Sci U S A. 2014; 111:4542–4547.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Advanced Magnetic Resonance Imaging for Pediatric Brain Tumors: Current Imaging Techniques and Interpretation Algorithms
- Proton therapy in pediatric brain tumors
- The role of chemotherapy in the treatment of pediatric brain tumors
- A Study of Pediatric Seizure Disorder by Brain Magnetic Resonance Image
- Basics for Pediatric Brain Tumor Imaging: Techniques and Protocol Recommendations